Covid-19 & the gut microbiota
Overview
By Pr. Tao Zuo
SYSU Research Institute of Gastroenterology, Guangdong Institute of Gastroenterology,The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Author
The gut microbiota, including the bacterial, fungal, and viral fractions, is co-populating the human intestines and regulating the host immu nity against pathogen invasions. The largely heterogeneous gut microbiota (GM) compositions across individuals may influence the host’s immune responses to SARS-CoV-2 infection, leading to various disease symptoms and outcomes of Covid-19. On the other hand, though SARS-CoV-2 infection primarily causes respirator y symptoms, it deeply dysregulates the host’s systemic immunity and impacts the gastrointestinal systems where the gut microbiota might be affected in both short and long term. Here, we review the current evidence on the impact of Covid-19 on the human GM as well as associations between GM composition and Covid-19 severity.
Covid-19 is a respiratory illness caused by a novel coronavirus (SARS-CoV-2) and is still affecting tens of millions of people worldwide today. Although most of Covid-19 patients present respiratory symptoms, up to 20% of them have gastrointestinal (GI) symptoms including diarrhea [1], suggesting that the digestive tract is an extrapulmonary site of disease expression and SARS-CoV-2 infection. In addition, Covid-19 presents a wide spectrum of disease severity, varying from asymptomatic, mild, severe, and up to critical resulting in respiratory failure or even death [2].
The GI tract is the largest immune organ in humans, playing critical roles in host defense against pathogens infections. Trillions of microorganisms live and colonize the human gut – bacteria, fungi, viruses, and other life forms that are collectively known as the microbiota – regulating the host immunity. Therefore, it is of paramount importance to understand if the gut microbiota modulates the host susceptibility to and severity of SARS-CoV-2 infection, as well as the impact of SARS-CoV-2 infection on the host GM and its downstream long-term effect on human health.
The gut bacterial microbiota and Covid-19
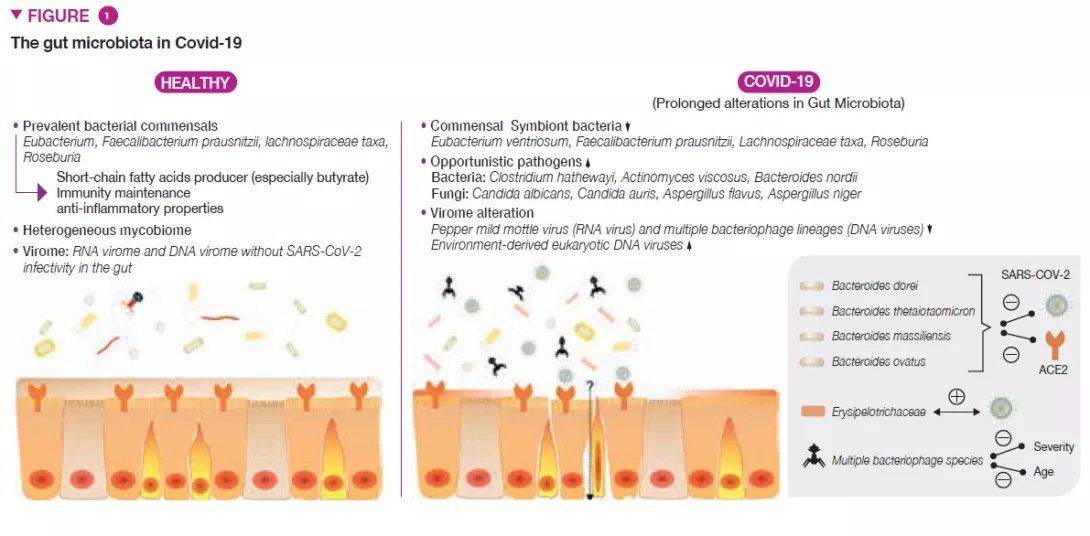
Covid-19 patients had significant alterations in the gut bacterial microbiome compared with healthy individuals, characterized by depletion of beneficial commensals and enrichment of opportunistic pathogens in the gut (Figure 1) [3]. Depletion of gut symbionts persisted even after the resolution of Covid-19. The baseline (at hospitalization) abundance of the bacteria Coprobacillus, Clostridium ramosum and Clostridium hathewayi showed positive correlation with Covid-19 severity, whereas there was an inverse correlation between the abundance of Faecalibacterium prausnitzii (known as an anti-inflammatory bacteria) and the disease severity.
SARS-CoV-2 uses the angiotensin converting enzyme 2 (ACE2) receptor to enter the host and this receptor is highly expressed in both the respiratory and gastrointestinal tracts [4]. ACE2 is important in controlling intestinal inflammation and gut microbial ecology [5]. Four Bacteroides species : B. dorei, B. thetaiotaomicron, B. massiliensis, and B. ovatus, were reported to inversely associate with ACE2 expression in murine gut [6]. Interestingly, their abundances in faecal microbiome also showed inverse correlation with faecal SARS-CoV-2 viral load in Covid-19 patients during the disease course. These findings suggest that the human bacterial GM is affected by Covid-19 and might calibrate the host defense against SARS-CoV-2 infection.
The fungal microbiome and Covid-19
The GI tract also harbors a large number of fungi, collectively known as the mycobiome (fungal microbiome), which have been shown to be causally implicated in GM assembly and immune development [7]. Patients with Covid-19 also had altered gut mycobiomes, characterized by enrichment of Candida albicans and highly heterogeneous mycobiome configurations (Figure 1) [8]. The diversity of the fecal mycobiome in patients with Covid-19 at discharge was 2.5-fold higher than that in healthy individuals. Opportunistic fungal pathogens, Candida albicans, C. auris, and Aspergillus flavus were highly present in faeces of Covid-19 patients during the disease course. Two respiratory symptom- associated fungal pathogens, A.flavus and A. niger, were detected in faecal samples from a subset of patients with Covid-19, even after disease resolution. Unstable gut mycobiomes and prolonged dysbiosis persisted in approximately 30% of patients with Covid-19.
The gut virome and Covid-19
Through shotgun RNA viral sequencing, a signature of active gut viral infection were found in 47% of patients with Covid-19, even in the absence of gastrointestinal symptoms and after respiratory clearance of SARS-CoV-2 [9], suggesting “quiescent” SARS-CoV-2 infection in the GI tract and potential faecal-oral transmission risk. Patients with such gastrointestinal SARSCoV- 2 activity harboured abnormal GM compositions and functions, featured by high abundances of opportunistic pathogens and enhanced capacity for biosynthesis of nucleotide and amino acid and carbohydrate metabolism (glycolysis) [9].
The human GI tract also harbours abundant viral/phage members collectively known as the gut virome. Covid-19 patients had under-representation of Pepper mild mottle virus (RNA virus) and multiple bacteriophage lineages (DNA viruses) and enrichment of environment-derived eukaryotic DNA viruses in faecal samples, compared to non-Covid-19 subjects (Figure 1) [10]. Faecal virome in SARSCoV- 2 infection showed more stress-, inflammation- and virulence-associated gene encoding capacities. At patient baseline, faecal abundances of the RNA virus, Pepper chlorotic spot virus, and multiple bacteriophage species inversely correlated with Covid-19 severity. These viruses were also inversely associated with blood levels of pro-inflammatory proteins, white cells and neutrophils, indicating gut resident viruses might tune host immune response to SARS-CoV-2 infection. Among Covid-19 severity-associated DNA virus species, 40% species showed inverse correlation with age, which may underlie the observation that elderly subjects are at a higher risk for a more severe Covid-19.
Conclusion
In summary, the collection of evidence suggests that the human GM (bacterial microbiota, mycobiome and virome) is impaired in Covid-19. Such dysregulation persists even after the disease resolution, which potentially pose a long-term health threat to the host. Gut microbiota composition is associated with host immune responses and Covid-19 severity to SARS-CoV-2 infection. Further research is needed to explore the long-term effects of Covid-19 and to improve the host GM and immunity to this unprecedented viral pandemic.







