Cholestasis impairs gut microbiota development and bile salt hydrolase activity in preterm neonates
COMMENTED ARTICLE - Children’s section
By Pr. Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Commentary on the original article by Lynch LE et al. Gut Microbes [1]
Cholestasis refers to impaired bile flow from the liver to the intestine. In neonates, cholestasis causes poor growth and may progress to liver failure and death. Normal bile flow requires an intact liver-gut-microbiome axis, whereby liver-derived primary bile acids are transformed into secondary bile acids. Microbial bile salt hydrolase (BSH) enzymes are responsible for the first step, deconjugating glycine- and taurine-conjugated primary bile acids. Cholestatic neonates often are treated with the potent choleretic bile acid ursodeoxycholic acid (UDCA), although interactions between UDCA, gut microbes, and other bile acids are poorly understood. This study was conducted on 124 stool samples collected from 24 preterm infants to find novel associations linking isomeric bile acids and BSH activity to neonatal growth trajectories. These data highlight deconjugation of bile acids as a key microbial function that is acquired in early neonatal development and is impaired by cholestasis.
What do we already know about this subject?
Preterm infants born before 37 weeks of amenorrhoea (WA) are at a greater risk of developing cholestasis. Cholestasis (i.e., impaired bile flow) is more likely in the presence of various risk factors such as prematurity, low birth weight and parenteral nutrition. In the absence of other causes, this is referred to as transient neonatal cholestasis. To improve cholestasis, ursodeoxycholic acid (UDCA) is often administered.
Bile acids are necessary for the absorption of lipids and fat-soluble vitamins. Reduced quantities of bile acids in the gut is observed in cholestasis, as are changes in the proportions of the different bile acids. Primary bile acids are produced from cholesterol and conjugated in the liver. Their interactions with the intestinal microbiota play an important role leading to the formation of secondary bile acids through the intermediary of a microbial enzyme, bile salt hydrolase (BSH).
Bile acids and intestinal microbiota have an impact on the growth and development of preterm infants. The authors looked at the impact of cholestasis on gut microbiota development and bile acid deconjugation in very premature neonates.
What are the main insights from this study?
The authors included 24 preterm neonates, 12 with cholestasis and 12 controls, born at 27.2 ± 1.8 WA, with a mean birth weight of 946 ± 249.6 g. Mean peak conjugated bilirubin was 7.0 mg/dL. No differences existed between the two groups in terms of intrauterine environment, delivery method and antibiotic use over time.
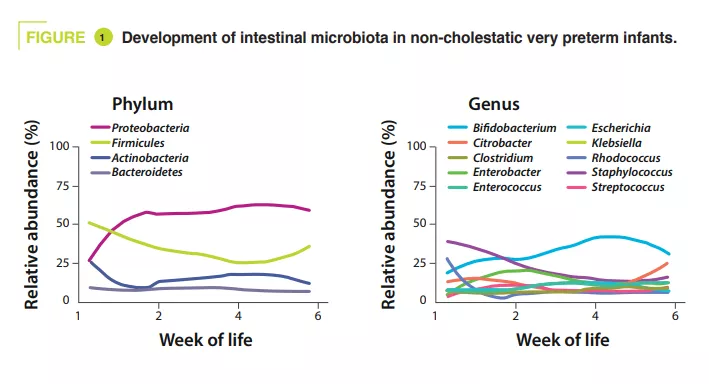
Stools were collected from birth to six weeks. Their sequencing (shotgun method) showed that in controls alpha-diversity increased during the first months of life. In terms of phyla, Proteobacteria and Firmicutes were the most abundant. In terms of genera, Staphylococcus was the most prominent at birth, its abundance then decreased while Klebsiella gradually increased in abundance (figure 1). Clostridium perfringens increased the most in relative abundance over time, based on postmenstrual age (PMA, which is the sum of postnatal age and WA at birth) (p = 0.01). According to the metagenomic analysis, the metabolic pathway most enriched in mature stools (32-40 weeks PMA) compared with less mature stools (25-28 weeks PMA) was secondary bile acid biosynthesis.
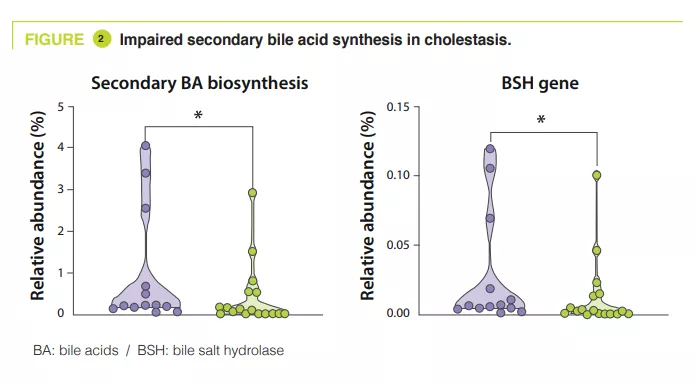
In the control group, the principal component analysis showed that the main factor influencing the composition of the intestinal microbiota was PMA, whereas it had no effect in cholestatic premature infants. Secondary bile acid biosynthesis was the most enriched pathway in stools from the control group relative to the cholestatic group at 32-40 weeks PMA (p = 0.04). Similarly, the authors observed a 55% reduction in the relative abundance of the BSH gene (p = 0.04) and Clostridium perfringens (p = 0.0008) in the cholestatic neonates (figure 2).
Faecal bile acid profile measured by mass spectrometry showed that the proportion of unconjugated bile acids increased from 4% at 25–28 weeks PMA to 98% by 32– 40 weeks PMA in the control group but was only 46% in cholestatic infants. However, it should be noted that some isomers may have a predictive value as they increased before the onset of cholestasis. UDCA used on five of the 12 premature neonates was found in their faeces at a concentration 522-fold higher compared to the seven other untreated neonates. UDCA administration increased the relative abundance of Firmicutes and decreased Proteobacteria (p < 0.05), with an enrichment in Clostridium perfringens at a species level.
Finally, premature neonates with faecal BSH gene abundance > 0.005% at 32–40 weeks PMA exhibited a 1.2-fold increased length and weight rate compared to those with an abundance < 0.005%. Equally, neonates with a faecal cholic acid composition of > 30% demonstrated increased length (14%), weight (18%), and head circumference (15.8%) (p < 0.05).
What are the consequences in practice?
This study offers an insight into the pathophysiological mechanisms that disrupt the liver-gut-microbiome axis during cholestasis. This opens the way to the possibility of correcting the enterohepatic cycle using (BSH-carrying) probiotics or other medicinal products.
CONCLUSION
In extremely preterm neonates, cholestasis disrupts the development of the intestinal microbiota, by reducing the acquisition of Clostridium perfringens and the capacity to synthesise secondary bile acids. In contrast, an increase in certain bile acids, linked to BSH activity, is associated with improved neonatal growth.

