Highlights from the 55th Espghan
By Dr. Tania Mahler
MD, Pediatric Gastroenterology & Nutrition - Chronic Pain & Functional Gastrointestinal Disorders - Qualifications in mindfulness and medical hypnosis for functional GI disorders. Clinical adjunct at Queen Fabiola Children’s University Hospital, Belgium
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
At the ESPGHAN (European Society of Pediatric Gastroenterology Hepatology and Nutrition) 2023 meeting held in Vienna, 4,300 participants from all over the world gathered. Following the challenging period of the Covid-19 pandemic, this event offered a revitalizing experience, as attendees were able to engage in live presentations and have faceto-face discussions This in-person interaction proved to be significantly more enjoyable and enriching than virtual alternatives. Various research groups focused their work on the gut microbiome and presented compelling data in the field of pediatrics.
The microbiome’s impact on health and disease is widely recognized, so it is evident that also pediatric clinicians and researchers try to get a better understanding of how we can manipulate the microbiome and how by using the signature of the microbiome we can detect disease in an early stage. This review aims to shed light on several key topics that have been extensively discussed.
Recommendations for the use of probiotics in selected pediatric gastrointestinal disorders
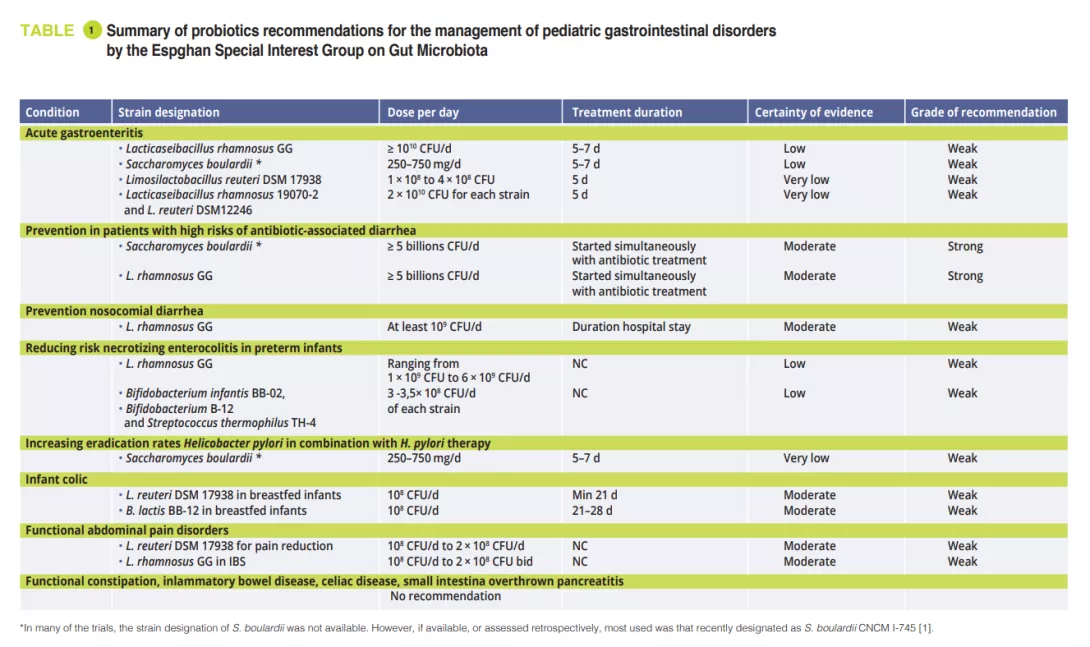
In February 2023, the Espghan special interest group on gut microbiota published recommendations for the use of probiotics for the management of selected pediatric gastrointestinal disorders based on systematic reviews and/or meta-analyses using the modified Delphi-process [1]. At the meeting of the special interest group on gut microbiota and modifications Prof. Szajewska showed us the results of this work. Only a few specific probiotic strains proof some utility in certain conditions. In the original paper [1] you find a clear overview of the actual recommendations.
FMT in adolescents suffering from refractory IBS
Dr De Bruijn from the group of Amsterdam UMC reported in the plenary session of the highest scoring abstract their study on the efficacy of FMT in adolescents with refractory IBS in a randomized double-blind placebo controlled trial [2]. Her talk was fascinating but also stirred a lot of reactions in the audience when she showed a slide from a patient receiving syringes with fecal material. To our knowledge only one other pediatric study has evaluated FMT to relieve abdominal bloating, another feature of disorders of the gut-brain interaction and which is often present in IBS [3].
The chronic pain in IBS can have an enormous impact on the functioning of children and adults leading to absenteeism at school and work and a poor quality of life. The origin of the disease is multifactorial and can best be explained by the biopsychosocial model. One of the key role factors is dysbiosis of the gut microbiota. In adults’ different studies have been published over the positive effect seen with FMT [4].
In pediatrics non-pharmacological treatments as education, hypnosis, mindfulness are more effective than pharmacological therapy [5]. Still in approximately 25% of patients’ symptoms persist. Pre-, pro-, and synbiotics are tested with various outcomes to correct the dysbiosis in IBS. Changing to FODMAP diet can also influence the gastrointestinal flora [5]. But in specific groups of patients FMT could be, if safe, the ultimate treatment to effectively restore a healthy gastrointestinal microbiome. In the study of De Bruijn et al., 32 patients with refractory IBS between 16 and 21 years old were recruited and randomized. One group received allogeneic (healthy donor) and the other group autologous (own) fecal infusions by nasogastric tube at baseline and 6 weeks later. Clinical efficacy was defined as the proportion of patients with a reduction of more than 50 points in the IBS Severity-Scoring-System (IBS-SSS). Patients were evaluated 12 weeks and 6 months after TMF. Both groups had similar IBSSSS at baseline. After the first evaluation there was no statistical difference, but at 6 months of follow-up there was improvement in 60% of the patients that received allogeneic TMF versus 25% in the autologous group (p = .048). Secondary outcome included health-related quality of life (QoL). Total QoL score at baseline did not differ between groups but became significantly better after allogeneic TMF. No adverse events were recorded. Allogeneic TMF seems an exciting way to treat refractory IBS in youth, but further studies are needed.
Microbiota and IBD
In the gastroenterology session on IBD a Czech group presented a study that had as aim to evaluate if the changes in microbiota in CD was due to the anti-TNFα treatment or was the result of decreased mucosal inflammatory activity [6]? Therefore, they compared children on anti-TNFα treatment with active Crohn’s disease (CD) and juvenile idiopathic arthritis (JIA). Their results showed that mucosal healing in CD was essential to obtain changes in the bacteriome. The antiTNFα treatment in JIA had no impact on the bacteriome of these patient group. Schwerd et al. followed 20 newly diagnosed pediatric CD patients treated first with exclusive enteral nutrition (EEN) with stool sampling [7]. Fifteen out of twenty patients went in remission. They demonstrated clear temporal and individual intestinal microbial and metabolite changes with reduced abundance of Lachnospiraceae and enriched unsaturated long chain fatty acids. Ex vivo fermentation with an EEN-like media and subsequent transfer in gnotobiotic mouse models showed a protective effect in contrast to the fiber-rich media and to those colonized directly with patient’s baseline microbiota. Based on those results they concluded that EEN-modulated patient microbiomes are regulating intestinal inflammation. They also elaborated on the possibility of using a low-fiber diet for long-term remission. A multicenter study in the UK (children and adults) studied the possibility to use a Crohn’s Disease TReatment-with-EATing (CD-TREAT) solid food diet to create a more palatable diet that could influence gut inflammation by changing the gut bacteria [8]. The diet is personalized for each patient but excludes specific dietary components such as gluten, lactose, alcohol. The 55% of patients adhering to this regime had a significant lower fecal calprotectin and had microbial and metabolic changes in the same line as patients under successful EEN. This was not seen in those not respecting the diet. Based on those findings, it could be interesting to use autologous feces of successfully treated EEN CD patients for FMT. The group of Schwerd analyzed this possibility using autologous capsule FMT. They concluded that this approach was unsuitable since there was still a to high pathogen burden and a to low microbiota diversity [9].
The Cologne group had a very interesting poster on the follow-up of 2 cases of very early IBD refractory to steroids and antiTNFα treatment. The first patient has ulcerative colitis and is now 3 years in total remission with weekly administered enema of donor stool preparation. The second patient with CD is only in partial remission after one year of follow-up [10].
Combined efforts of scientific researchers and clinicians will further unravel the mystery of the gut microbiota and will eventually bring new ways to treat and to prevent diseases.
1. Szajewska H, Berni Canani R, Domellöf M, et al. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J Pediatr Gastroenterol Nutr 2023; 76: 232-47
2. De Bruijn C, Zeevenhoven J, Vlieger A, et al. Efficacy of fecal microbiota transplantation in adolescents with refractory irritable bowel: a randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr 2023; 76(S1 Suppl 1): 1-1407
3. Wang YZ, Xiao FF, Xiao YM, et al. Fecal microbiota transplantation relieves abdominal bloating in children with functional gastrointestinal disorders via modulating the gut microbiome and metabolome. J Dig Dis 2022; 23: 482-92
4. El-Salhy M, Winkel R, Casen C, et al. Efficacy of Fecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology 2022; 163: 982-94.e14
5. Mahler T, Hoffman I, Smets F, et al. The Belgian consensus on irritable bowel syndrome: the paediatric gastroenterologist view. Acta Gastroenterol Belg 2022; 85: 384-6
6. Hurych J, Mascellani Bergo A, Lerchova T, et al. The faecal microbiome and metabolome changes in Crohn’s disease are associated with decreased mucosal inflammatory activity. J Pediatr Gastroenterol Nutr 2023; 76 (S1 Suppl 1): 1-1407
7. Schwerd S, Häcker D, Siebert K, et al. Exclusive enteral nutrition initiates protective functions in the gut microbiota and metabolome to induce remission in pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr 2023; 76 (S1 Suppl 1): 1-1407
8. Macdonald J, Wilson D, Henderson P, Din S, e Chantges in faecal microbiome and metabolome are more pronounced in Crohn’s disease patients who adhered to the CD-TREAT diet and responded by calprotectin. J Pediatr Gastroenterol Nutr 2023; 76 (S1 Suppl 1): 1-1407
9. Hölz H, Heetmeyer J, Tsakmaklis A, et al . Autologous fecal microbiota transfer in pediatric Crohn ́s disease patients under treatment with exclusive enteral nutrition harbors major challenges - a feasibility test. J Pediatr Gastroenterol Nutr 2023; 76 (S1 Suppl 1): 1-1407
10. Fritz T, Huenseler C, Broekaert I. Safety and efficacy of long-term faecal microbiota transfer in very early onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2023; 76 (S1 Suppl 1): 1-1407

