The infant's gut at the heart of immunity
By Dr Travis J. De Wolfe
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Authors
Table of contents
Table of contents
Development of innate immune barriers
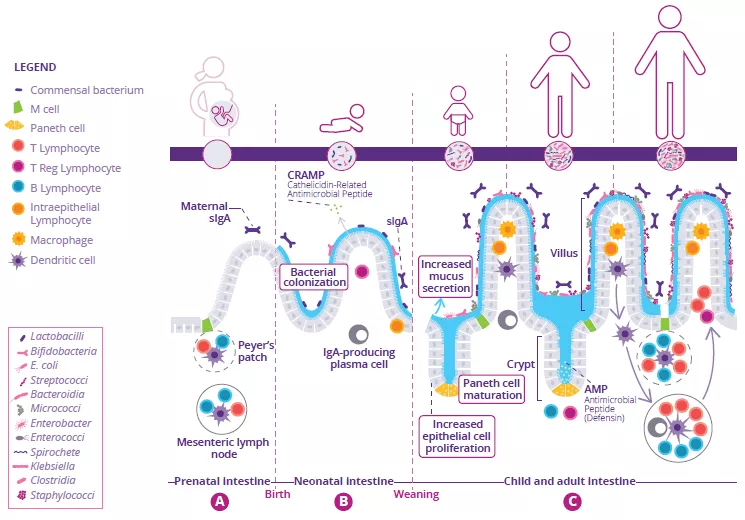
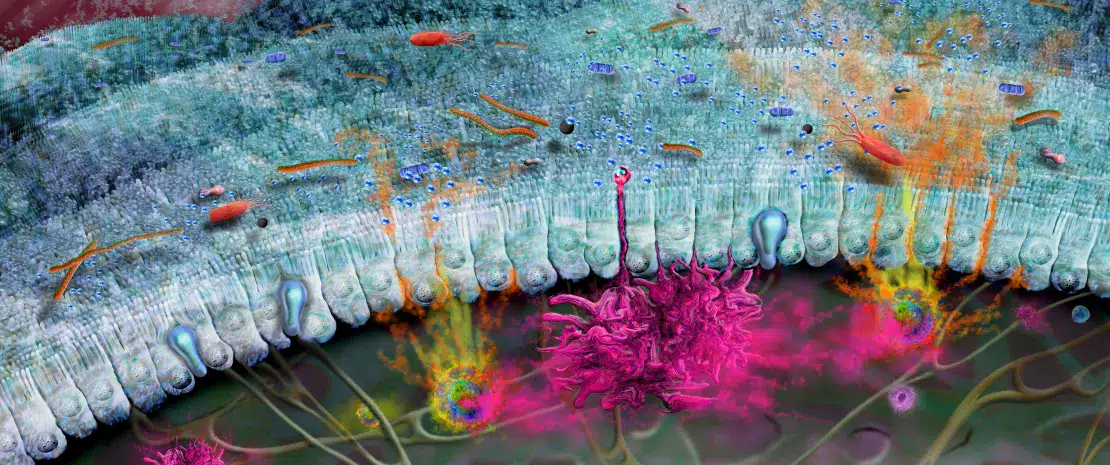
Development of the intestinal immune system begins before birth and continues throughout neonatal weaning. In utero, immature lymphoid structures including Peyer’s patches and mesenteric lymph nodes are generated (Fig 1A). Since these structures are not fully functional until later in development, to compensate, antimicrobial peptides (AMP) are produced by the gut epithelium and function as a defense barrier in response to the first bacterial colonizers (Fig 1B).1 Mucus is another important barrier structure which is produced by goblet cells and secreted at the apical surface of the GI tract. Together, these innate immune barriers play a key role in limiting direct contact of the gut microbiota with host epithelial cells, especially as the microbiota establishes itself within the infant intestine.
80% At least 80% of the body Ig-producing cells are located in the gut.
Neonatal adaptive immune system is also critical during development
Immunoglobulin A (IgA) are produced with varying affinity toward members of the microbiota as well as specific food antigens ingested by the neonate. Secreted IgA act to cross-link these targets in the intestinal lumen and limit their ability to adhere to and/or penetrate the gut epithelium (Fig 1B).2 Correspondingly, during weaning, the neonatal gut microbiota becomes increasingly diverse and concentrated in response to a changing diet and development of crypt-villous architecture. This requires further protection of the epithelial barrier through maturation of local lymphoid structures. Activated Paneth cells begin to produce host defense proteins (defensins) at the base of small intestinal crypts, allowing other epithelial cells to transition away from producing AMP at baseline. Lastly, proliferation of epithelial cells increases alongside the increased secretion of mucus (Fig 1C).
FIGURE 1: Development of gut microbiota and intestinal immune system before birth (A), before (B) and after weaning (C).
Adapted from Brandtzaeg P, 20173 and Ximenez C et al, 20176
Importance of intestinal homeostasis
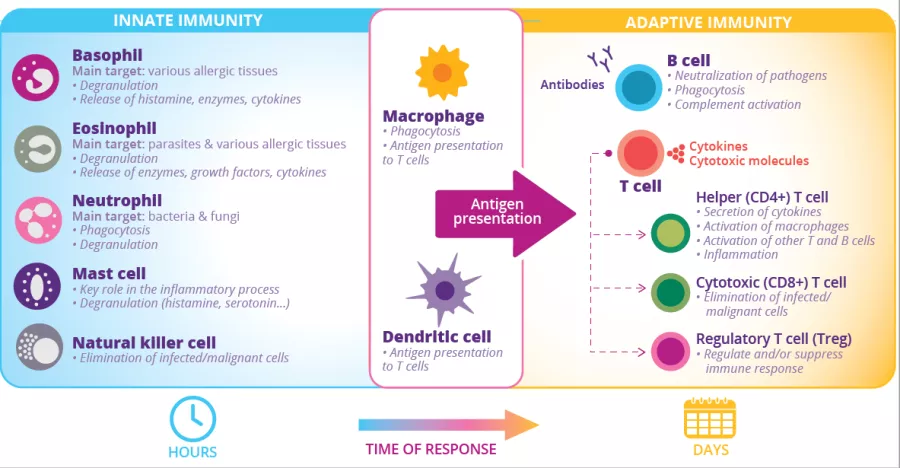
At least 80% of the body’s Ig-producing cells are located in the gut:3 this is the largest effector organ of humoral immunity. Specialized antigen-sampling epithelial cells (M cells) have a gatekeeping function by facilitating the transport of antigens - arising from commensal bacteria, diet or pathogens - from the gut lumen to the underlying lymphoid cells. These antigens will then be digested by dendritic cells (DC) and presented to the adaptive immune system.
Together, the different components of intestinal immunity promote homeostasis through two anti-inflammatory strategies (Fig 1C):
1) Immune exclusion of foreign antigens limits/prevents the gut microbiota from colonizing or penetrating the intestinal mucosa. This is performed by sIgA.3
2) Oral tolerance acts to limit local and peripheral immune responses to innocuous antigens that come in contact with the epithelial barrier.4 This depends on Treg cells with regulatory functions (Fig 2).3
When these strategies are operating properly, immune system regulation along with the actions of commensal microbiota in the development and training of this system will lead to the establishment of a durable and homeostatic host-commensal relationship that has long-term implications for human health.5
Beyond immune cells: the importance of intestinal mucus barrier
by Dr. Larissa Celiberto
The gut is lined by a single layer of cells, called the intestinal epithelium, over top of which lies a dense mucus layer (Fig 1). Together these barriers confine microbes within the gut lumen, as well as protect the underlying immune system from unnecessary activation by the microbiota.3 Intestinal mucus generate and release the mucin 2 (MUC2), a sugar-coated glycoprotein that provides structure to the mucus. Recent studies have shown that the maturation and function of the mucus layer are strongly influenced by the gut microbiota, while the types of sugars found on MUC2 can also influence which bacteria are able to bind to it or use it, and its sugar chains as a nutrient source.7 Notably, a mucus barrier that is disrupted or dysfunctional can lead to increased penetration or passage of potentially harmful bacteria out of the lumen (e.g. leaky gut), resulting in systemic infection and inflammation.8 Moreover, a defective mucus layer, and a corresponding gut microbiota dysbiosis,9 has been observed in several diseases (such as inflammatory bowel disease (IBD),10,11 diabetes…12) thus highlighting the importance of this protective barrier to human health.
FIGURE 2: Innate and adaptive immune cells and functions.
3 Brandtzaeg P. (2017) Role of the Intestinal Immune System in Health. In: Baumgart D. (eds) Crohn's Disease and Ulcerative Colitis. Springer, Cham.
4 Commins SP. Mechanisms of Oral Tolerance. Pediatr Clin North Am. 2015 Dec;62(6):1523-9.