Role of the gut microbiota in immune regulation
By participating in the regulation of innate and adaptative immune responses, the gut microbiota becomes one of the pillars of defense mechanisms, especially thanks to the presence of specific bacteria called “segmented filamentous bacteria” (SFB).3 The microbiota is able to act on immunity, and its composition and diversity can in turn be controlled by that same immune system.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Innate lymphoid cells (ILC) are a type of specialized innate immune cells. They are a group of lymphocytes that do not have antigen-specific receptors6 and have been recently identified and divided into three groups based on the type of secreted cytokines: ILC1 that produces interferon gamma (IFN-γ) and similar to T-helper cells Th1, ILC2 similar to Th2 (IL-5, IL-6, IL-13) and ILC3 similar to Th17 (IL-17, IL-22). The studies on the microbiota and ILCs, currently booming, also show that the microbiota seems to be necessary to the development and functions of ILCs, especially Group 3 ILCs. ILC3 are the main intestinal source of IL-22, a cytokine that is key to the production of antimicrobial proteins.6
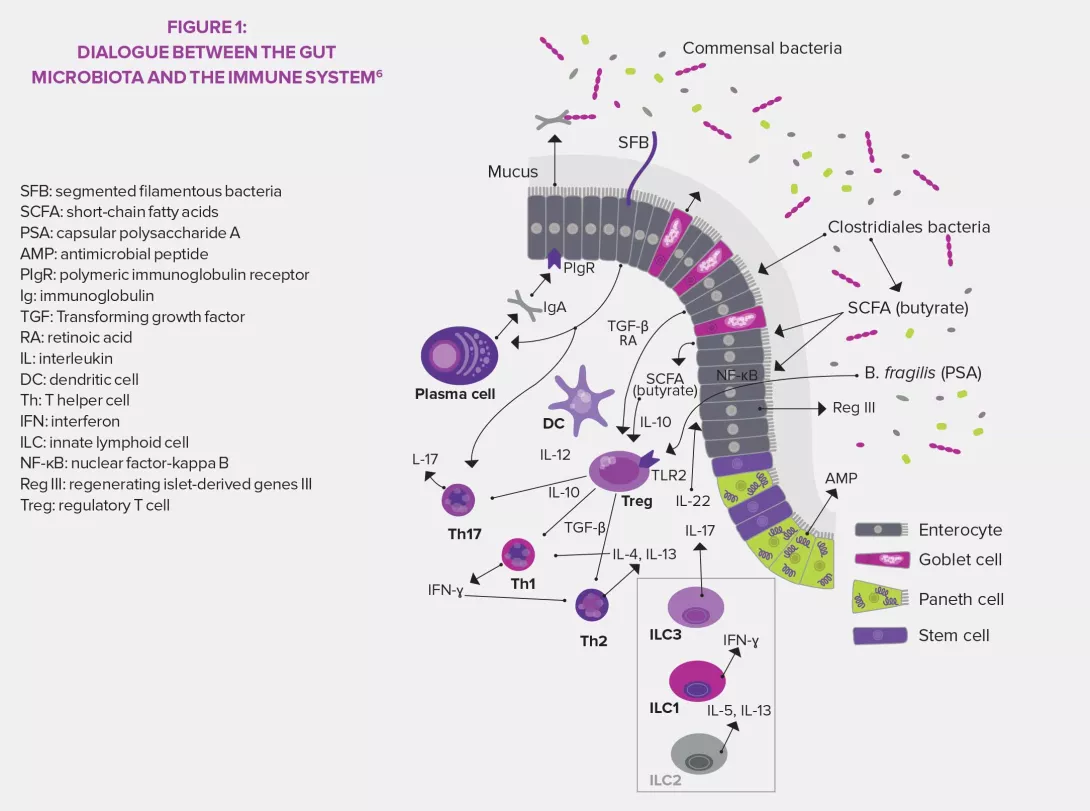
KEY FACTS SEGMENTED FILAMENTOUS BACTERIA (SFB)
Commensal bacteria (Clostridiales order) previously identified in vertebrate animals and detected in humans thanks to molecular sciences.3,5
They are necessary to the maturation of the intestinal and pulmonary immune barrier and induce the production of IgA and the activation of proinflammatory and regulatory T-cells.3,5
They have a protective effect against type 1 diabetes mellitus (non-obese diabetic [NOD] mouse model), pneumonia caused by methicillin-resistant Staphyloccocus aureus (MRSA), and some bacterial (Citrobacter rodentium) and parasitic (Entamoaba histolytica) infections.3,5
They can have adverse effects by promoting the development of autoimmune diseases in autoimmune encephalitis or arthritis models.3,5
ADAPTIVE IMMUNITY
The gut microbiota is also core to the activation of the adaptive response. At the T-cell level, it induces the maturation of naïve T-cells into IL-17-producing cells (Th17) which stimulate the production of antimicrobial peptides by the intestinal epithelium. It also promotes the synthesis of some CD4+ regulatory T-cells (Treg) that have an anti-inflammatory action. Finally, it contributes to the development of secondary lymphoid tissues in the intestines where T-cells are stored. Regarding B-cells, the gut microbiota ensures, through IL-17, that secretory IgA produced by B-cells crosses through the intestinal mucosa to join the lumen and neutralize harmful toxins and bacteria. The combination of strong IgA responses and pro-inflammatory (Th17) and anti-inflammatory (Treg) responses from T-cells create a state of physiological inflammation controlled by the gut microbiota.3,5
6 Marteau P, Dore J. Gut microbiota: a full-fledged organ, Chapter 8 «Gut microbiota and immune system». John Libbey Eurotext. 2017.