Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signalling in mice
COMMENTED ARTICLE - ADULTS’ SECTION
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Author
52% Just 1 in 2 people having suffered from a digestive condition involving the microbiota had made the connection
Comment on the article by De Palma et al. Science Translational Medicine 2022 [1]
Gut microbiota has been implicated in chronic pain disorders, including irritable bowel syndrome (IBS), yet specific pathophysiological mechanisms remain unclear. In this article, the authors showed that decreasing intake of fermentable carbohydrates improved abdominal pain in patients with IBS, and this was accompanied by changes in the gut microbiota and decreased urinary histamine concentrations. Germ-free mice colonised with faecal microbiota from patients with IBS were used to investigate the role of gut bacteria and the neuroactive mediator histamine in visceral hypersensitivity. Germ-free mice colonised with the faecal microbiota of patients with IBS who had high urinary histamine developed visceral hyperalgesia and mast cell activation. When these mice were fed a diet with reduced fermentable carbohydrates, the animals showed decreased visceral hypersensitivity and mast cell accumulation in the colon. The authors then observed that faecal microbiota from patients with IBS with high urinary histamine produced large amounts of histamine in vitro. The authors identified Klebsiella aerogenes, carrying a histidine decarboxylase gene variant, as a major producer of this histamine. This bacterial strain was highly abundant in the faecal microbiota of three independent cohorts of IBS patients compared with healthy individuals. Pharmacological blockade of the histamine 4 receptor in vivo inhibited visceral hypersensitivity and decreased mast cell accumulation in the colon of germ-free mice colonised with the high histamine-producing IBS faecal microbiota. These results suggest that therapeutic strategies directed against bacterial histamine could help treat visceral hyperalgesia in a subset of IBS patients with chronic abdominal pain.
WHAT DO WE ALREADY KNOW ABOUT THIS SUBJECT?
Gut microbiota has been implicated in the pathophysiology of some chronic pain disorders, including pain associated with irritable bowel syndrome (IBS) and fibromyalgia [2]. This assumption is largely based on studies reporting an association between pain levels and changes in the composition of gut microbiota, on differences in pain thresholds between conventionally bred and germ-free mice, which return to normal after bacterial colonisation, and on the ability of bacteria to produce neuroactive metabolites in vitro [3]. However, there is a lack of data demonstrating causality, the precise mechanisms involved in visceral pain induced by the gut microbiota, or identifying the specific bacterial species involved. The authors of this article previously reported that abdominal pain in IBS patients improved after restriction of fermentable carbohydrate intake. This improvement was associated with changes in gut microbiota profiles and lower concentrations of urinary histamine [2], a known mediator implicated in visceral hypersensitivity [4]. In this article, the authors investigated gut microbiota functions triggering histamine production and visceral hypersensitivity using germ-free mice colonised with the faecal microbiota of IBS patients or healthy individuals.
KEY POINTS
- Gut microbiota is involved in chronic pain in IBS patients
- In the context of a diet rich in fermentable carbohydrates, some bacteria in the microbiota, including Klebsiella aerogenes, contribute to histamine production
- Histamine produced by the microbiota plays a role in visceral hypersensitivity by boosting mast cell recruitment by activation of the H4 receptor
- Pharmacological blockade of the histamine 4 receptor in vivo inhibits visceral hypersensitivity and reduces mast cell accumulation in the colon of germ-free mice colonised with the high histamine-producing IBS faecal microbiota. These results suggest that therapeutic strategies directed against bacterial histamine could help treat visceral hyperalgesia in a subset of IBS patients with chronic abdominal pain
WHAT ARE THE MAIN INSIGHTS FROM THIS STUDY?
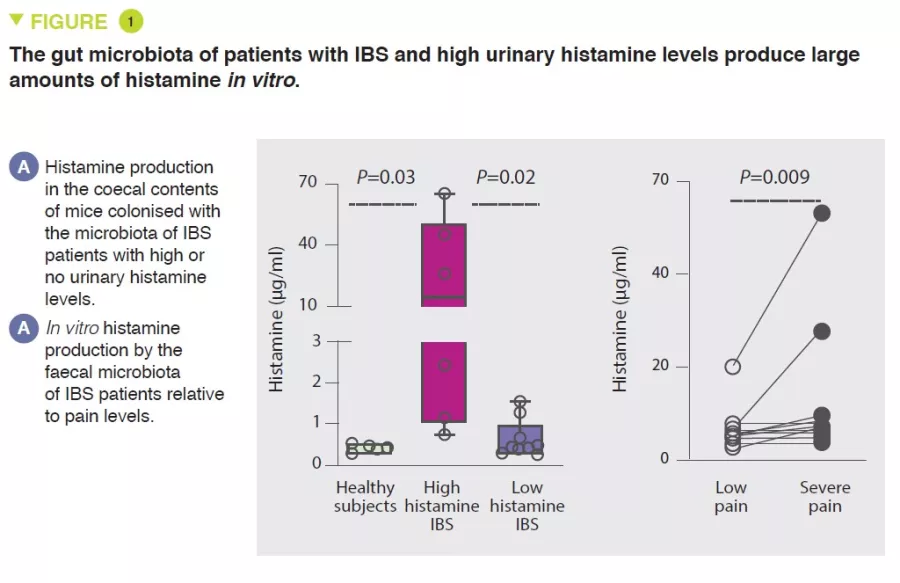
A positive correlation was first observed between visceral pain severity and urinary histamine concentration in a cohort of IBS patients.
Visceral hypersensitivity and gut mechanosensation, evaluated using action potential measurements in colonic afferent nerves, was higher in germ-free mice colonised with faecal microbiota from IBS patients with high urinary histamine levels compared with mice colonised with microbiota associated with low urinary histamine levels. It was shown that the microbiota was responsible for the production of histamine in IBS patients with high urinary levels of this metabolite (Figure 1). In addition, a diet low in fermentable carbohydrates reduced histamine-mediated visceral hypersensitivity.
Using culturomics, Klebsiella bacteria were then identified as the main producer of histamine in IBS patients with elevated urinary levels of this molecule.
Compared with healthy subjects, IBS patients had a higher prevalence of K. aerogenes and a higher relative abundance of the histidine decarboxylase (hdc) gene, responsible for histamine production. Mechanistically, histamine produced by K. aerogenes was implicated in mast cell recruitment, which plays a role in the pain phenotype in mice. H4R (histamine receptor 4) expression was elevated in the colon of mice colonised with the faecal microbiota of IBS patients with high levels of urinary histamine. In vitro, H4R blockade blocked mast-cell chemotaxis. Finally, in vivo, H4R blockade reduced visceral-motor responses to colorectal distension in mice colonised with the faecal microbiota of IBS patients with high levels of urinary histamine.
WHAT ARE THE CONSEQUENCES IN PRACTICE?
This study reveals the specific role of histamine production by some bacteria in the gut microbiota in the painful symptoms suffered by a subgroup of IBS patients, in the context of a diet rich in fermentable carbohydrates. It suggests that gut distension related to gas production is not the main nociceptive trigger in these patients. Identifying K. aerogenes, or other histamine- producing bacteria, could help guide dietary recommendations as well as therapies targeting the microbiota or the use of H4 receptor antagonists in this subgroup of IBS patients.
Conclusion
Gut microbiota is involved in visceral pain in IBS patients. In a subset of patients, it is linked to histamine production in the context of a diet rich in fermentable carbohydrates. Targeting histamine-producing bacteria or blocking the H4 receptor could offer a therapeutic strategy to such patients.
1. De Palma G, Shimbori C, Reed DE, et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci Transl Med 2022 ; 14 : eabj1895.
2. McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017 ; 66 : 1241-51.
3. Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014 ; 5 : 381–9.
4. Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 2007 ; 117 : 636-47.