The Gut-Brain axis
By Pr. Sarkis K. Mazmanian, John W. Bostick, Nadia Suryawinata
Biology and Biological Engineering, California Institute of Technology, Pasadena, CA, USA
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Comment on the article by Gabanyi et al. Science 2022 [1]
The microbiota affects metabolism and recent data has implicated gut bacteria in feeding behaviors in mice. A challenge in the field is to define gut-brain pathways that link microbial compounds to neuronal processes that impact appetite. In this study, Gabanyi and co-workers identified a functional role for Nod2, a pattern recognition receptor for bacterial muropeptides, bacterial cell wall components, in regulating appetite and thermoregulation in aged, female mice. The authors found that muropeptides accumulate in the brains of aged mice and regulate the activity of arcuate hypothalamic inhibitory neurons. Targeted Nod2-deficiency in these neurons results in increased appetite, weight gain, and reduced body temperature response that is dependent on the presence of the microbiota. These results suggest that the regulation of neuronal activity by Nod2 signaling in the brain affects complex behaviors in mice and warrants further investigation.
WHAT DO WE ALREADY KNOW ABOUT THE SUBJECT?
Food intake is essential to the survival of animals, and the inappropriate regulation of feeding behavior leads to serious metabolic and psychiatric consequences, such as obesity and anorexia [2]. Food intake involves complex processes from nutrient processing and uptake in the gut and its periphery to the central nervous system that regulates appetite and drives feeding. Much focus in the field of appetite biology has centered on the characterization of neural circuits involved in feeding, such as the agouti-related peptide (AgRP)-expressing neurons in the arcuate hypothalamus that are necessary for homeostatic feeding [3]. More recently, the gut and its resident microbes have been shown to regulate metabolism [4] and aspects of feeding behavior [5]. Whether compounds produced by microbes are influencing appetite remains less well-established. Short chain fatty acids as a byproduct of microbial fermentation reduce food intake in mice [6].
However, a gut-brain pathway that links microbial compounds to neuronal processes that regulate appetite and feeding behavior has not been previously shown. The microbial pattern recognition receptor Nod2 is suggested to mediate feeding, as Nod2 knockout mice shows enhanced weight gain when fed a high fat diet [7]. Furthermore, the downstream signaling component of Nod2, nuclear factor kB (NFkB), is expressed in neurons of the hypothalamus, and its hypothalamic activation regulates energy balance [8]. This suggests that the hypothalamus may present a unique integration point for signals derived from the microbiome and feeding behaviors.
WHAT ARE THE MAIN INSIGHTS FROM THIS STUDY?
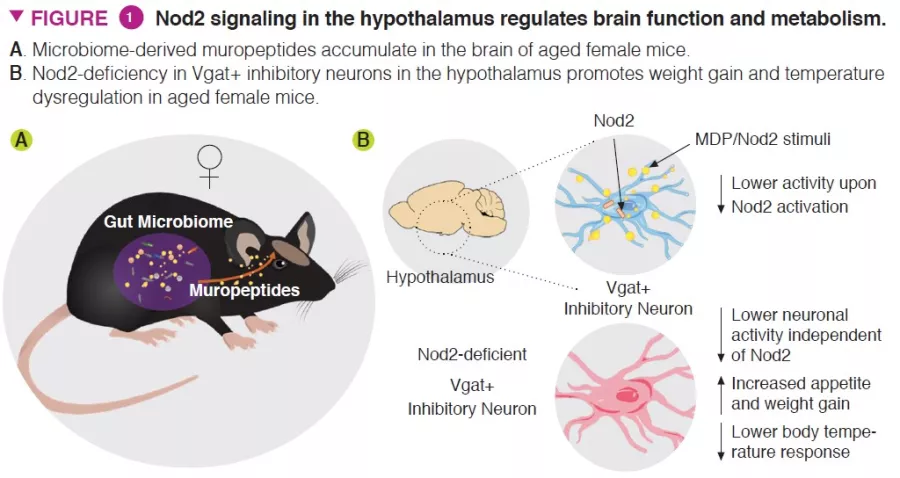
The authors demonstrated that activation of Nod2 signaling in the hypothalamus affected feeding and thermoregulatory behavior in mice (Figure 1). Nod2 was found to be expressed in neurons in multiple regions of the mouse brain, including the striatum, thalamus, and hypothalamus. The authors then investigated whether radiolabeled muropeptides could reach the brain, when introduced through the gastrointestinal tract directly or via radiolabeled bacteria. Both methods of delivery resulted in the accumulation of muropeptides in the brain.
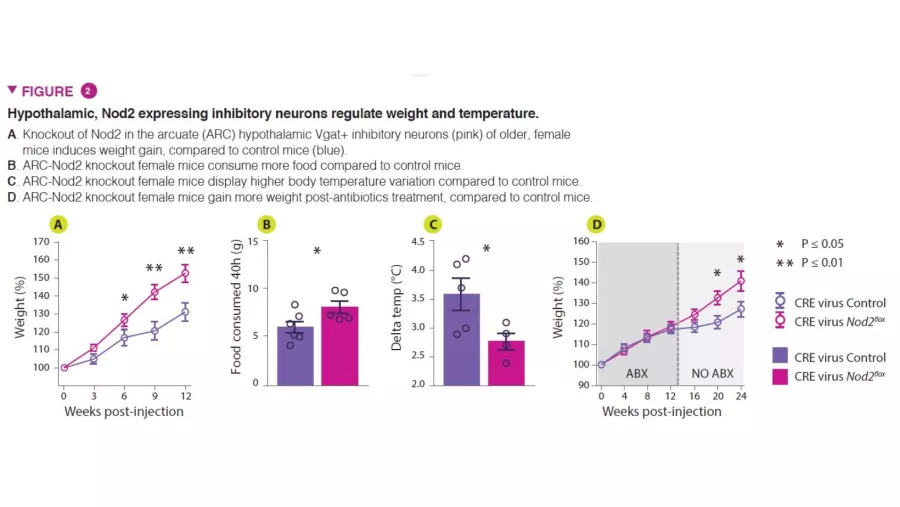
To investigate the functional role of Nod2 in neurons, conditional knockout mouse models that targeted Nod2 for deletion were utilized to show that older female mice with Nod2 deleted in inhibitory neurons expressing the vesicular GABA transporter (Vgat/ Slc32a1) display increased weight gain and dysregulated temperature control. Measurement of Fos expression in the brain revealed that older female mice have higher neuronal activity in the arcuate and dorsomedial regions of the hypothalamus. Next, the authors injected Cre-expressing adeno-associated viruses (AAVs) into Nod2flox mice to knockout Nod2 expression locally in inhibitory neurons of the arcuate hypothalamus, demonstrating that Nod2 deficiency in hypothalamic neurons was sufficient to cause weight changes and temperature dysregulation (Figure 2).
Finally, to examine the role of the microbiota in the Nod2-dependent changes in appetite and temperature regulation, the authors treated hypothalamic neuron-specific Nod2 knockout mice with broad-spectrum antibiotics. Hypothalamic Nod2-deficent mice that underwent antibiotic treatment display normal appetite and weight gain until the antibiotics are removed, at which point they exhibit increased appetite and weight gain compared to Nod2-sufficient control mice. This data suggests that microbiota- derived products can modulate appetite in female mice via a Nod2-dependent mechanism.
Key Points
- Nod2 is expressed in neurons in multiple regions of the mouse brain, including the striatum, thalamus, and hypothalamus
- Nod2 ligands, such as muropeptides, accumulate in the brains of aging mice
- The activity of hypothalamic inhibitory neurons is regulated by Nod2 expression
- Appetite and temperature regulation is perturbed in Nod2-deficient aged female mice
WHAT ARE THE CONSEQUENCES IN PRACTICE?
In this interesting new work, Gabanyi et al. identified a functional role for Nod2 expression in neurons of the hypothalamus in regulating appetite and temperature in aging female mice, but not in male mice. The cellular and molecular mechanisms that determine this effect remain to be elucidated. Sex differences in microbiome composition may play a role in the dissimilarities in response to neuronal Nod2 deficiency; however, microbial composition was not investigated by the authors. Moreover, in addition to muropeptides, other microbe-derived products and endogenous stimuli can regulate the expression or activation of Nod2 [9], though are not addressed in this study. Additional data is needed to distinguish the activity and contribution of these alternative stimuli from muropeptides. Other possible contributors to the outcomes reported in the paper may include the increased gut and blood-brain barrier permeability that occurs with age, which may allow more microbe-derived molecules to enter the circulation from the gut and accumulate in the brain [10]. Further investigation is required to clarify the roles of sex and age in the observed phenotypes.
Conclusion
This study reports that Nod2- deficiency in hypothalamic neurons is sufficient to induce changes in appetite and temperature regulation in aged female mice. Replication in mice and future work in humans is needed to validate these exciting findings.
1. Gabanyi I, Lepousez G, Wheeler R, et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 2022; 376: eabj3986.
2. Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell 2015; 161: 133-45.
3. Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 2015; 160: 829-41.
4. Zarrinpar A, Chaix A, Xu ZZ, et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 2018; 9: 2872.
5. Yu KB, Hsiao EY. Roles for the gut microbiota in regulating neuronal feeding circuits. J Clin Invest 2021; 131: 143772.
6. Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014; 5: 3611.
7. Rodriguez-Nunez I, Caluag T, Kirby K, Rudick CN, Dziarski R, Gupta D. Nod2 and Nod2-regulated microbiota protect BALB/c mice from diet-induced obesity and metabolic dysfunction. Sci Rep 2017; 7: 548.
8. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008; 135: 61-73.
9. Kuss-Duerkop SK, Keestra-Gounder AM. NOD1 and NOD2 Activation by Diverse Stimuli: a Possible Role for Sensing Pathogen-Induced Endoplasmic Reticulum Stress. Infect Immun 2020; 88: e00898-19.
10. Mossad O, Batut B, Yilmaz B, et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat Neurosci 2022; 25: 295-305.