Diarrhea and the role of microbiota
By Dr. Sanda Maria Cretoiu
Department of Morphological Sciences, Cell and Molecular Biology and Histology, “Carol Davila” University of Medicine and Pharmacy Bucharest, Romania
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Intestinal disorders can manifest symptoms such as frequent and loose stools, known as diarrhea. This signal from the digestive system can occur for many reasons, from infections and reactions to certain foods to adverse reactions to medications and pre-existing health conditions - summarized in [1]. The intestinal microbiota, i.e., the totality of microorganisms present in the intestine, is essential for the preservation of digestive health and for its impact on the functioning of the intestine. Recent studies illustrate the link between the microbiota and diarrhea of diverse etiology. A balanced and diverse microbiota is vital for overall digestive health, nutrient absorption, and immune system regulation. Currently, there is a tendency towards the large-scale introduction of ways to reprogram the intestinal microbial community: prebiotics, probiotics and postbiotics or the transplantation of fecal matter in order to prevent or treat diarrhea. Research on microbiota modulation will offer actionable strategies for diarrhea prevention and treatment in the near future. The following overview covers the main diarrheal illnesses related to dysbiosis and some aspects regarding microbiota management to ameliorate these gastrointestinal afflictions.
The relationship between the microbiota and diarrhea
Diarrhea can involve various mechanisms (table 1), and the majority of them are related to the role of microbiota:
- Protection of the microbial balance, this state, known as eubiosis, is fundamental for the health of the human body because it prevents and slows down the expansion of pathogens. Disturbance of the balance between the main microbial strains, known as dysbiosis, can increase susceptibility to infections and contribute to diarrhea. The literature generally indicates that diarrhea represents a major dysbiosis and that the degree of dysbiosis is related to the etiology and the stage of diarrhea [6]. Following acute diarrhea, the taxonomy of the microbiota changes a lot. In early stages of diarrhea, facultative fastgrowing anaerobes such as Proteobacteria (mostly Enterobacteriaceae/Escherichia coli) and Streptococcus (mainly Streptococcus salivarius and Streptococcus gallolyticus) dominate and favor the drastic disappearance of obligate anaerobic gut commensals (Blautia, Prevotella, Faecalibacterium, Lachnospiraceae, Ruminococcaceae, etc.) [2, 3]. The consequence is that short-chain fatty acid (SCFA) also decreases, and the integrity of the intestinal barrier starts to be under threat, possibly leading to gut permeability. In the recovery phase after diarrhea, a proposed model shows that in the midstage, there is an abundance of Bacteroides (the 7th day since disease onset). At the same time, in the late-stage Prevotella and SCFA-producing Firmicutes dominate [4, 5].
- Protection against pathogenic invaders. The microbial community of the gut microbiota competes for resources, produces antimicrobial substances, and acts as a barrier against enteropathogens. Beneficial bacteria in the gut, such as certain strains of Bifidobacteria and Lactobacilli, have been demonstrated to have beneficial effects on infectious diarrhea caused by rotavirus in young children. However, there are no clinical trials to demonstrate it [6]
- Regulating the immune system. The gut microbiota helps educate and modulate immune responses, promoting tolerance to harmless substances and defending against pathogens. Dysregulation of the immune response due to microbiota imbalances can contribute to inflammation and diarrhea. After antibiotics for Clostridioides difficile-induced diarrhea, such as vancomycin, a reduced relative abundance of Bacteroidetes and Firmicutes is observed, while Proteobacteria and Fusobacteria increase and leading to a decrease in SCFA propionate, creating premises for inflammation [7]
- Maintenance of gut function and metabolism. Beneficial bacteria ferment dietary fibers to produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. SCFAs contribute to maintaining a healthy intestinal lining, promote water absorption, and provide an energy source for colonocytes. Imbalances between bacterial strains may impact these functions, leading to functional diarrhea due to decreased SCFA production. Increasing its production enhances colonic fluid absorption. [8]
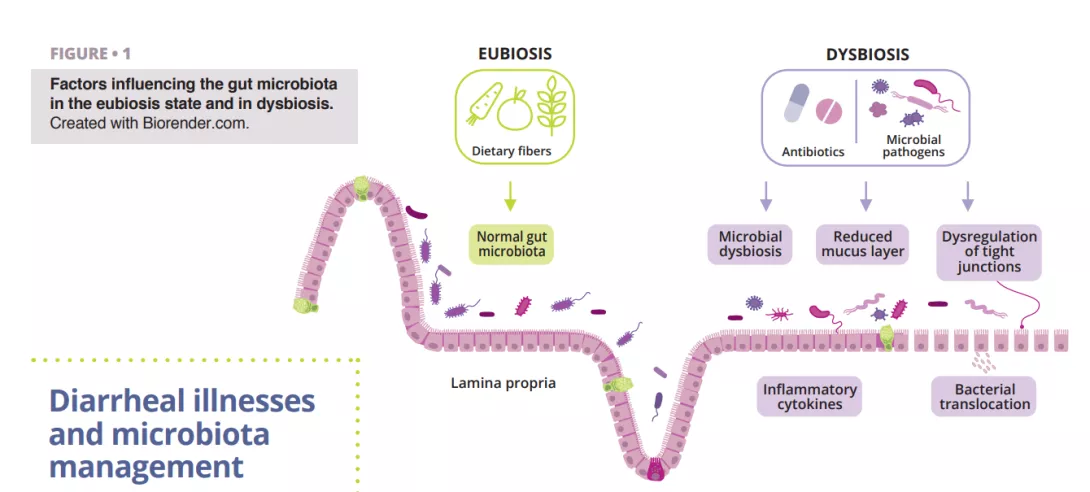
Diarrheal illnesses and microbiota management
Infectious diarrhea
Bacterial, viral or parasitic gut infections cause acute diarrhea and are frequently spread through contaminated water. Most cases of diarrhea are improved in a few days, but severe diarrhea can lead to serious dehydration and can become lethal [9].
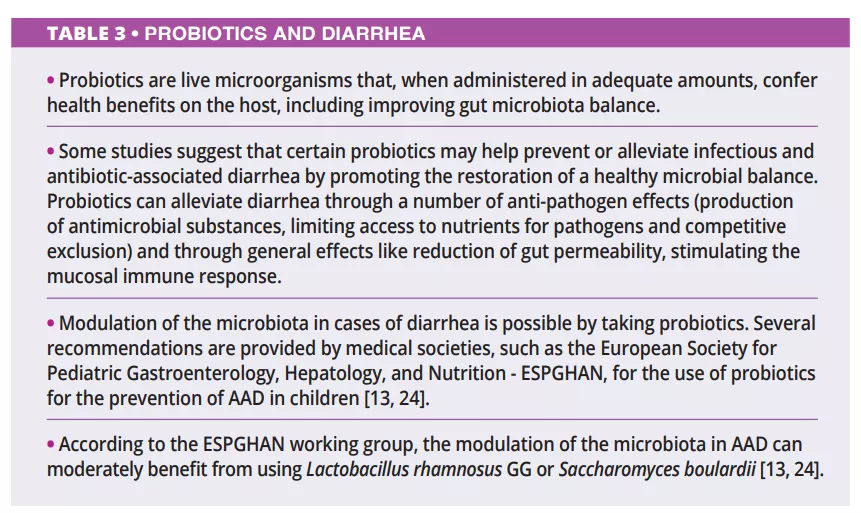
Rotaviruses remains the primary cause of diarrhea-associated deaths in children [11], and management of this viral disease generally involves oral or intravenous hydration, tailored to the severity of dehydration [12]. Furthermore, based on the latest conclusions from the ESPGHAN committee (2023) [13], healthcare providers might suggest certain probiotic strains for acute gastroenteric episodes in children, acknowledging their potential (certainty of evidence: low; grade of recommendation: weak) to decrease the duration of diarrhea, and/or hospital stay, and/or volume of fecal discharge. However, a randomized, double-blind, controlled trial of Bolivian children with acute rotavirus diarrhea demonstrated a decreased duration of diarrhea by using an oral rehydration solution plus a mixture of probiotics by comparison with simple rehydration solution [11].
Travelers diarrhea
More than 60% of the adults from developed countries who travel to developing countries experience acute diarrhea, also known as traveler’s diarrhea (TD). The most frequently identified pathogens implicated in traveler’s diarrhea episodes are Escherichia coli, Campylobacter jejuni, Salmonella species and Shigella species. Thus, the recommended treatment strategies include antibiotic therapy with azithromycin or fluoroquinolones for moderate to severe cases [14]. However, antibiotics are not recommended to prevent TD, due to insufficient evidence of their prophylactic efficacy and partially due to the risk of antibiotic resistance [15].
There is conflicting data regarding the efficacy of probiotics in preventing traveler’s diarrhea [16]. One systematic review and meta-analysis compared the efficacy of rifaximin and probiotics in preventing TD. [15].
Antibiotic-associated diarrhea
Antibiotics are one of the most prescribed medications and represent an effective treatment for several infectious pathologies [17]. One of the complications associated with antibiotic therapy is antibiotic-associated diarrhea (AAD), which occurs in 5%-35% of the patients who receive antibiotherapy [18]. AAD can be defined as three or more watery or loose stools per day for at least two consecutive days, which is strictly related to antibiotics administration and no other cause [14]. The highest risk is attributed to aminopenicillins, cephalosporins and clindamycin, which primarily target anaerobes [19].
The lack of an infectious agent identified in AAD may be explained by the direct toxic effect of the antibiotics on the intestinal mucosa, which may cause diarrhea. Due to their beneficent properties, probiotics are now being researched and used for both treatment and prophylaxis of AAD [16, 18].
Clostridium difficile associated diarrhea
Clostridioides difficile (CD) infection is the most common cause of nosocomial antibiotic-associated diarrhea in adults. Risk factors include age over 65 years, long hospitalization in intensive care, and administering antibiotics (fluoroquinolones, clindamycin, cephalosporins, and betalactams in particular) or proton pump inhibitors. During antibiotherapy, anaerobes that produce SCFAs may disappear due to antibiotic-induced alterations in the gut microbiota, which may also disturb the metabolism of carbohydrates and bile and cause an osmotic imbalance. Following antibiotic intake, all three intestinal barriers are affected: the epithelial intestinal cells, the mucus and antimicrobial peptides layer, and the immunoprotective layer composed of different immune cells and various biomolecules (figure 1). This event can interfere with the production of mucin, cytokines, and antimicrobial peptides, dysregulating intestinal function and leading to other infections or even causing recurrent episodes of infections. The American Gastroenterological Association (AGA) conditionally recommends specific probiotics for preventing CD infection in individuals on antibiotics, noting that the quality of evidence is low [20].
Emerging discoveries and the future of diarrhea management
Recent breakthroughs in microbiota research, including metagenomic analysis and microbial transplantation, are revolutionizing our approach to diarrhea treatment (figure 2).
Treatment options for diarrhea should take into account the causative mechanisms involved in the genesis of diarrhea, from infectious toxins capable of disrupting fluid and electrolyte balance to patients who developed dysbiosis due to other causes and patients with large amounts of non-absorbed carbohydrates in the lumen triggering osmotic diarrhea.
There is limited data regarding the prebiotics and fibers in treating diarrhea (table 2). Apparently, prebiotics are more prone to prevent and treat the recurrence of diarrhea. At the same time, fibers, mainly the viscous ones, are more indicated during acute episodes due to their water-retaining capacity. Other therapeutic options involve, in some cases, the probiotic administration and (table 3), in severe cases, the use of fecal microbiota transplantation (FMT).
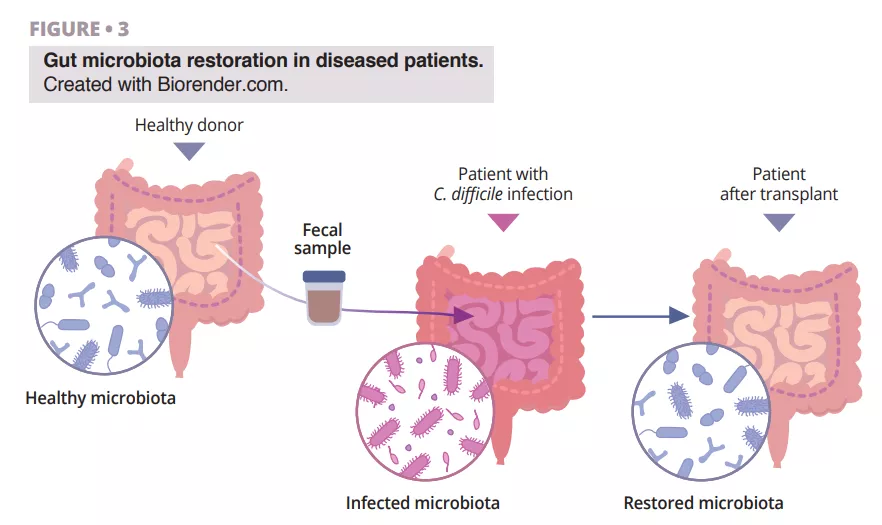
The fascinating journey of FMT discovery has roots in ancient China, where Ge Hong treated patients with severe diarrhea using a “yellow soup” consisting of feces suspension. In modern times, Dr. Ben Eiseman used fecal enemas from healthy individuals to treat pseudomembranous enterocolitis back in 1958. Nowadays, there is growing interest in fecal microbiota transplantation (FMT) as a treatment for recurrent Clostridioides difficile infection (CDI), which points out its utility [22]. Research is ongoing regarding its efficacy towards inflammatory bowel disease, diabetes, cancer, liver cirrhosis, and brain diseases such as Parkinson’s [23]. The benefits of using FMT in patients with diarrhea are based on the idea that the healthy microbial flora introduced via FMT has the ability to outcompete pathogens and restore the composition of a healthy gut microbiome (figure 3).
Conclusion
Research reveals that reduced gut microbiota diversity is associated with increased susceptibility to diarrhea, paving the way for potential diagnostic and therapeutic interventions. Maintaining a balanced and diverse gut microbiota prevents diarrhea and promotes overall digestive health. Imbalances in the microbiota, known as dysbiosis, can result from infectious acute diarrhea or dysbiosis due to other factors (frequent antibiotic use, unhealthy diet, malabsorption) that can contribute to chronic diarrhea. Understanding the complex interplay between microbial composition and clinical symptoms is crucial for personalized patient management of diarrhea. Tailored approaches based on unique microbiota profiles can lead to more effective strategies or interventions. The introduction of probiotics and a diet rich in prebiotics, microbiota transplantation, integration of multi-omics approaches, innovative use of machine learning, and the growing trend of interdisciplinary research collaborations may help restore microbial balance and support gastrointestinal well-being. Hopefully, in the future, one could design microbiome-based therapies as suggested by Peter J. Turnbaugh, laying the base for new treatment principles [25].
1. Iancu MA, Profir M, Roşu OA, et al. Revisiting the Intestinal Microbiome and Its Role in Diarrhea and Constipation. Microorganisms 2023; 11: 2177.
2. David L, Weil A, Ryan ET, et al. Gut microbial succession follows acute secretory diarrhea in humans. mBio 2015; 6: 1-14.
3. Sohail MU, Al Khatib HA, Al Thani AA, et al. Microbiome profiling of rotavirus infected children suffering from acute gastroenteritis. Gut Pathog 2021; 13: 21.
4. Becker-Dreps S, Allali I, Monteagudo A, et al. Gut Microbiome Composition in Young Nicaraguan Children During Diarrhea Episodes and Recovery. Am J Trop Med Hyg 2015; 93: 1187-93.
5. Cannon JL, Seabolt MH, Xu R, et al. Gut Microbiome Changes Occurring with Norovirus Infection and Recovery in Infants Enrolled in a Longitudinal Birth Cohort in Leon, Nicaragua. Viruses 2022; 14: 1395.
6. Azagra-Boronat I, Massot-Cladera M, Knipping K, et al. Strain-Specific Probiotic Properties of Bifidobacteria and Lactobacilli for the Prevention of Diarrhea Caused by Rotavirus in a Preclinical Model. Nutrients 2020; 12: 498.
7. Kim AH, Lee Y, Kim E, et al. Assessment of oral vancomycin-induced alterations in gut bacterial microbiota and metabolome of healthy men. Front Cell Infect Microbiol 2021; 11: 629438.
8. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 2010; 72: 297-313.
9. Collinson S, Deans A, Padua-Zamora A, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2020; 12:CD003048.
10. Desselberger U. Viral gastroenteritis. Medicine 2017; 45: 690-4.
11. GBD 2016 Diarrheal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18: 1211-8.
12. Iturriza-Gómara M, Cunliffe NA. 34 - Viral Gastroenteritis. Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP. (eds) Hunter’s Tropical Medicine and Emerging Infectious Diseases (tenth edition). Elsevier, 2020, pp. 289-307.
13. Szajewska H, Berni Canani R, Domellöf M et al.; ESPGHAN Special Interest Group on Gut Microbiota and Modifications. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J Pediatr Gastroenterol Nutr 2023; 76: 232-47.
14. Kopacz K, Phadtare S. Probiotics for the Prevention of antibiotic-associated diarrhea. Healthcare 2022; 10: 1450.
15. Fan H, Gao L, Yin Z, et al. Probiotics and rifaximin for the prevention of travelers’ diarrhea: A systematic review and network meta-analysis. Medicine 2022; 101: e30921.
16. Girardin M, Seidman EG. Indications for the use of probiotics in gastrointestinal diseases. Dig Dis 2011; 29: 574-87.
17. Goodman C, Keating G, Georgousopoulou E, et al. Probiotics for the prevention of antibiotic-associated diarrhoea: a systematic review and meta-analysis. BMJ Open 2021; 11: e043054.
18. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 2008; 3: 563-78.
19. Barbut F, Meynard JL. Managing antibiotic associated diarrhoea. BMJ 2002; 324: 1345-6.
20. Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020; 159: 697-705.
21. Codex AC. Report of the 31th session of the codex committee on nutrition and foods for special dietary uses. Rome, Italy: FAO/WHO 2009.
22. Peery AF, Kelly CR, Kao D, et al.; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on Fecal Microbiota-Based Therapies for Select Gastrointestinal Diseases. Gastroenterology 2024; 166: 409-34.
23. Tariq R, Disbrow MB, Dibaise JK, etal. Efficacy of Fecal Microbiota Transplantation for Recurrent C. Difficile Infection in Inflammatory Bowel Disease. Inflamm Bowel Dis 2020; 26: 1415-20.
24. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr 2014; 59: 132-52.
25. Rock RR, Turnbaugh PJ. Forging the microbiome to help us live long and prosper. PLoS Biol 2023; 21: e3002087.

