Gut microbiota from post-Covid-19 patients induces lung inflammation and brain dysfunction in mice [1]
Viviani Mendes de Almeida
Laboratory of Microbiota and Immunomodulation - Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais - UFMG, Belo Horizonte, Brazil
Angélica Thomaz Vieira
Laboratory of Microbiota and Immunomodulation - Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais - UFMG, Belo Horizonte, Brazil
Daiane Fátima Engel
Department of Clinical Analysis, School of Pharmacy, Universidade Federal de Ouro Preto - UFOP, Ouro Preto, Brazil and Center for Social and Affective Neuroscience, Linköping University, Linköping Sweden
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Viviani Mendes de Almeida
is a PhD student under Pr. Angélica Thomaz Vieira’s supervision. Viviani Mendes was selected from the special call of paper of the Microbiota Mag. She gives us a tour from her recent publication about the influence of microbiota in post-Covid effects. Her study was recently published in Gut Microbes [1].
What do we already know about this subject?
Covid-19 has wreaked havoc on a global scale, resulting in millions of confirmed cases and fatalities as of March 2023. Long-term complications of Covid-19 are pervasive, affecting even individuals with mild or asymptomatic cases. Among pathophysiological responses triggered by Sars-CoV-2 infection, several studies have linked gastrointestinal symptoms and altered gut microbiota in Covid-19 during and after the infection. On SARS-CoV-2 infection, growing evidence supports the role of gut microbiota in influencing Covid-19 severity and post-Covid effects [2]. Dysbiosis, an imbalance in the gut microbiota composition, is a critical factor in the development of various diseases. Severe Covid-19 cases have been associated with alteration of the intestinal microbiota that may persist for up to a year following the initial infection [3, 4]. However, until now, it was known that Covid-19 can alter the composition of the intestinal microbiota, but we were unaware of the causal effects that the post-Covid microbiota can have on the host’s physiology.
What are the main insights from this study?
Microbiota analysis of 72 individuals with a history of Covid-19 (post-Covid group) and 59 healthy controls showed no significant differences in gut microbiota diversity (α and β diversity) between the groups, while post-Covid subjects exhibited a higher prevalence of Enterobacteriaceae strains with drug-resistant phenotypes. A higher proportion of post-Covid individuals reported antibiotic use, likely due to Covid-19 treatment. Importantly, Klebsiella strains, associated with antimicrobial resistance (AMR), were notably increased in post-Covid gut microbiota (figure 1).
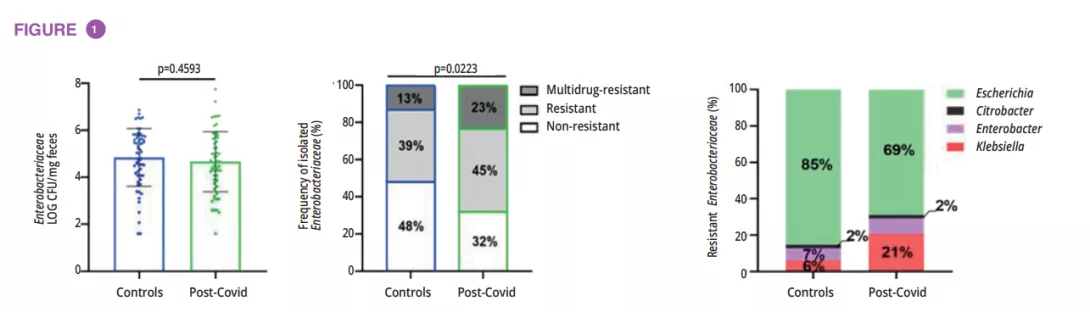
To understand the direct contribution of post-Covid microbiota to the host, fecal microbiota transplantation (FMT) was performed in germ-free mice using samples from post-Covid and control donors. Post-Covid mice exhibited lung inflammation (figure 2A).
They were also more susceptible to infection with multidrug-resistant Klebsiella pneumonia displaying a more severe lung pathology and inflammatory cell infiltration but were less efficient at clearing the bacteria. Increased Enterobacteriaceae levels in the blood of post-Covid mice suggested systemic translocation. In addition, reduced serum acetate levels were observed in post-Covid Klebsiella pneumonia-infected mice (figure 2A).
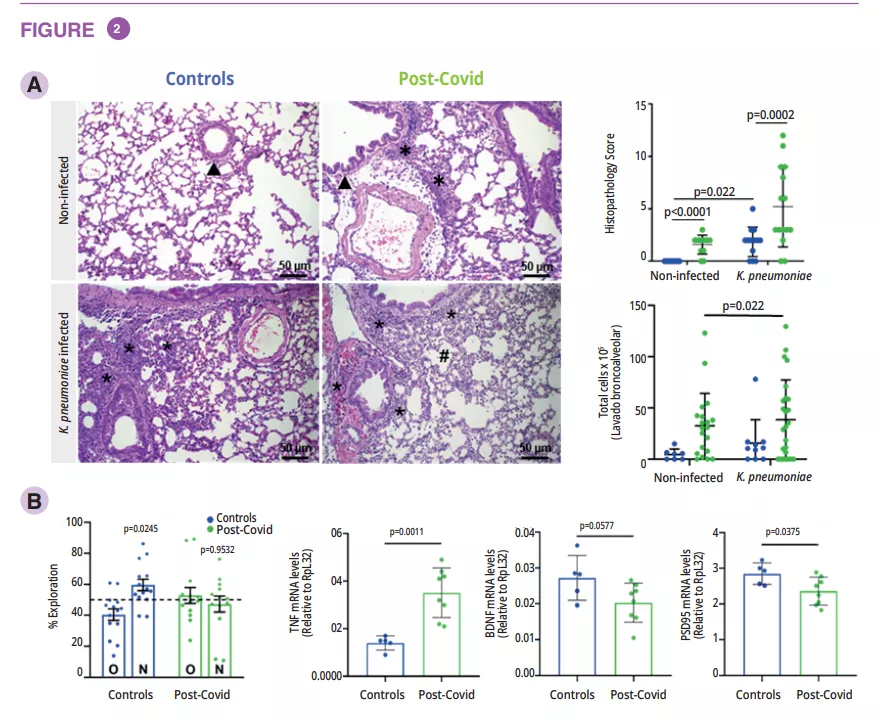
Post-Covid mice exhibited memory impairment in cognitive behavioral tests, along with increased TNF expression and decreased neuroprotective factors in the hippocampus (figure 2B). Administration of a strain probiotic to mice infected with a murine coronavirus prevented memory impairment, reduced weight loss and lung tissue inflammation.
What are the consequences in practice?
This study warns about the relationship between Covid-19 and the global burden of antimicrobial resistance. Furthermore, it highlights for the first time the causal effect of post-Covid microbiota on lung and nervous system alterations.
- Enterobacteriaceae strains with an antibiotic resistance phenotype are highly present in the intestinal microbiota of post-Covid subjects
- Transplanted mice with post-Covid samples showed lung inflammation and difficulty dealing with a pulmonary infection by multidrug-resistant Klebsiella pneumoniae
- Transplanted mice with postCovid samples also exhibited cognitive performance impairment, even after viral clearance
CONCLUSION
The study provides compelling evidence that gut microbiota from individuals following SARS-CoV-2 infection, even after viral clearance, can lead to lung inflammation, cognitive impairment, and increased susceptibility to secondary infections in mice. It highlights the potential for microbiome-based interventions, such as probiotics, to mitigate post-Covid sequelae.
1. Mendes de Almeida V, Engel DF, Ricci MF, et al. Gut microbiota from patients with Covid-19 cause alterations in mice that resemble post-Covid symptoms. Gut Microbes 2023; 15: 2249146.
2. Zuo T, Liu Q, Zhang F, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with Covid-19. Gut 2021; 70: 276-84.
3. Chen Y, Gu S, Chen Y, et al. Six-month follow-up of gut microbiota richness in patients with Covid-19. Gut 2022; 71: 222-5.
4. Liu Q, Mak JWY, et al. Gut microbiota dynamics in a prospective cohort of patients with postacute Covid-19 syndrome. Gut 2022; 71: 544-52.

