Gut Microbiota #21
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Gut microbiota as predictor of acute pancreatitis severity
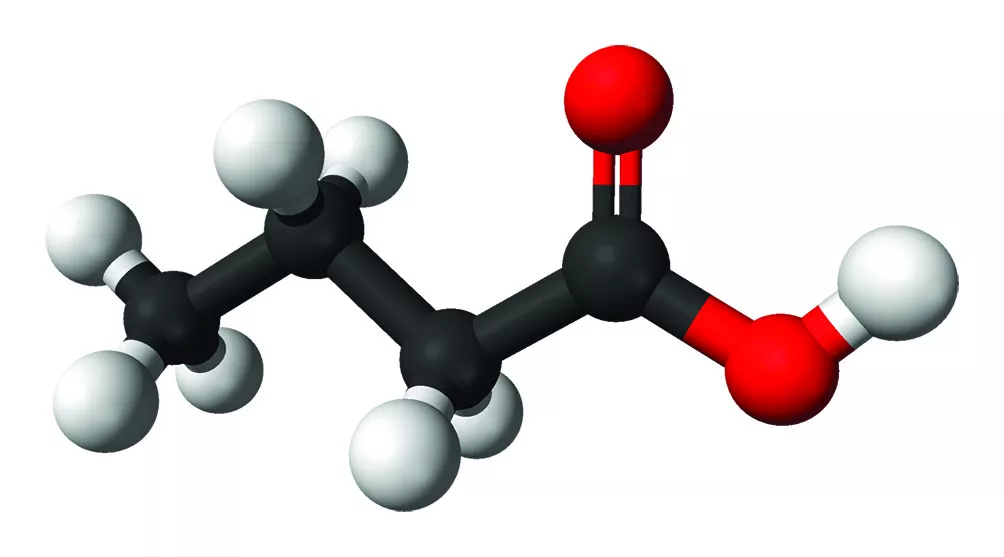
Severe acute pancreatitis (AP) patients are at risk of elevated mortality, for which determining the course of disease within the first few hours would be very important. The current complex scoring systems cannot predict AP severity early enough, and thus, novel markers are needed. While there seem to be a bilateral link between AP and gut microbiome, larger prospective clinical studies have been lacking. This paper presents results of orointestinal microbiome from 450 patients with AP from 15 European centers. The samples were sequenced by full-length 16S rRNA and metagenomic sequencing using Oxford Nanopore. The revised Atlanta classification (RAC) redefines severity of AP into three categories: mild, moderate, and severe (RAC I-III, respectively). This study found that Bray-Curtis distance of the rectal microbiomes was different in RAC III compared with RAC I and RAC II. Further, several bacterial species were differentially abundant depending on the RAC category. Bray-Curtis distances were also different between alive and deceased patients in rectal but not buccal microbiomes. In addition to mortality, the length of hospital stay associated with early alterations of rectal microbiome. In the end, the authors found that 16 bacterial species were differentially abundant in severe vs. non-severe AP. In Ridge regression, these species together with systemic inflammatory response syndrome could faithfully predict disease severity. Interestingly, all these species are producers of short-chain fatty acids (SCFA). Accordingly, functional pathways of SCFA production were more expressed in severe AP. While the finding is intriguing, it is still unknown whether SCFA producing bacteria are cause or consequence of severe AP.

Links between gut microbiome in type 2 diabetic Emirates
The incidence of type 2 diabetes (T2D) is increasing drastically in Middle East countries. Several Western studies have shown the contribution of gut microbiome in T2D-associated insulin resistance and low-grade inflammation, but studies in Middle East populations are scarce. Further, the existing studies show inconclusive results of how the microbial community composition and functions contribute to the pathogenesis of T2D. To gain more insight, the authors analyzed stool samples of 84 individuals from the United Arab Emirates with or without T2D using nanopore metagenomic sequencing. Unlike many earlier Western studies, this study reported no differences in gut microbiota alpha-diversity between healthy controls and T2D. Further, after correcting for multiple comparisons, the authors did not find differential abundance of any microbial species or KEGG orthology (KO) features between the groups. However, a gene set enrichment analysis revealed 8 functions with higher abundance in the control group and 5 in the T2D group. These differentially abundant modules associated with the degradation of amino acids, such as arginine, the degradation of urea and homoacetogenesis. These functions seem to have pro-inflammatory effects, and thus, may contribute to low-grade inflammation, a hallmark of T2D. Ultimately, the authors used prediction analysis to identify 3 potential biomarkers of T2D. These included a depletion of Enterococcus faecium and Blautia as well as an enrichment of Absiella spp or Eubacterium limosum in T2D. Interestingly, E. faecium is shown to have lipid-lowering and anti-obesity effects, and therefore, might partly contribute to the pathogenic T2D phenotype. To conclude, this study was successful in identifying specific microbial biomarkers, including functions and taxa that may help in predicting the development of specific T2D-associated disease conditions.

Microbial butyrate inhibits immunosuppressive factors in gastric cancer
Gastric cancer (GC) is one of the leading causes of cancer death worldwide. Early detection is important for successful treatment of GC. Programmed death-ligand 1 (PD-L1), a target of cancer immunotherapy, is highly expressed in tumor-associated macrophages that can be regulated by the gut microbiome. One possible way through which the microbiome may have anti-cancer effects is the production of short-chain fatty acids, including butyrate. In this study, advanced GC patients expressed more immunosuppressive markers, namely PD-L1 and interleukin (IL)-10, in macrophages, dendritic cells and cancer mucosa than healthy controls. The gut microbiota of the GC patients was characterized by lower diversity and dysbiosis. At genus level, lower abundances of butyrate-producing bacteria, such as Faecalibacterium and Bifidobacterium were detected in GC patients. Interestingly, administration of butyrate and Faecalibacterium into the peripheral blood mononuclear cells of GC patients decreased the number of PDL1- and IL-10-expressing macrophages. In addition, butyrate suppressed the growth on cultured GC cells. However, it remained unclear which Faecalibacterium strain was used in the in vitro experiment. Ultimately, a humanized tumor mouse model was injected with GC cells and peripheral blood mononuclear cells of from healthy controls or GC patients with or without butyrate. The experiment showed that butyrate significantly decreased tumor size and the immunosuppressive markers PD-L1 and IL-10. Thus, butyrate may have therapeutic potential via suppressing cancer cell growth in GC.