Children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorder share distinct microbiota compositions
COMMENTED ARTICLE - Children’s section
By Pr.Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Commentary on the original article by Bundgaard-Nielsen et al. [1]
An association has been suggested between altered gut microbiota, and attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD), respectively. Thus, the authors analyzed the gut microbiota composition in children and adolescents with or without these disorders and evaluated the systemic effects of these bacteria. They recruited study participants diagnosed with ADHD, ASD, and comorbid ADHD/ASD, while the control groups consisted both of siblings and non-related children. The gut microbiota was analyzed by 16S rRNA gene sequencing of the V4 region, while the concentration of lipopolysaccharide-binding protein (LBP), cytokines, and other signaling molecules were measured in plasma. Importantly the gut microbiota compositions of cases with ADHD and ASD were highly similar for both alpha- and beta-diversity while differing from that of non-related controls. Furthermore, a subset of ADHD and ASD cases had an increased LBP concentration compared to non-affected children, which was positively correlated with interleukin (IL)-8, 12, and 13. These observations indicate disruption of the intestinal barrier and immune dysregulation among the subset of children with ADHD or ASD.
What do we already know about this subject?
Attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are neurodevelopment disorders. These children with ADHD and ASD often have gastrointestinal disorders such as abdominal pain and constipation. Genetic abnormalities are implicated in the onset of these disorders, coupled with interaction with environmental risk factors, dietary intake in particular. For this reason, in parallel with drug therapies, dietary management is proposed, the composition of the gut microbiome being essential in the regulation of the gut-brain axis. Moreover we know that children with ASD are often selective eaters which may explain a modification of the gut microbiota. In addition to the dysbiosis, an increase in intestinal permeability has been described, in addition to low grade systemic inflammation, in both ADHD and ASD. The aim of this study was to analyse modifications to the gut microbiota in the groups ADHD, ASD and those with both ADHD/ASD, compared with healthy siblings and non-related controls. The secondary objectives were to assess intestinal permeability and the immune system.
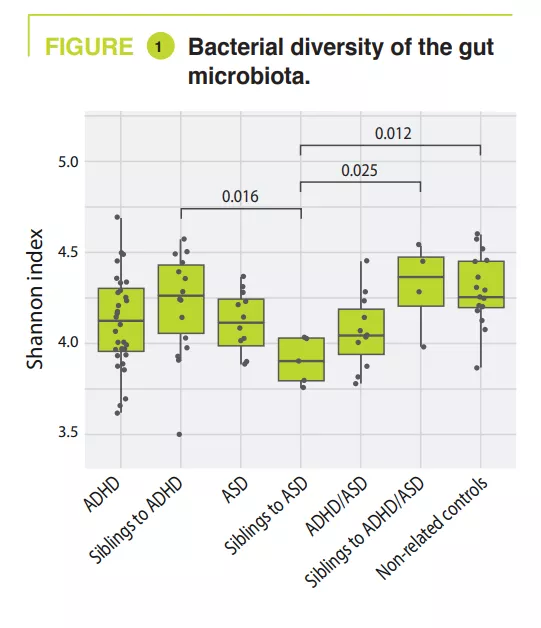
What are the main insights from this study?
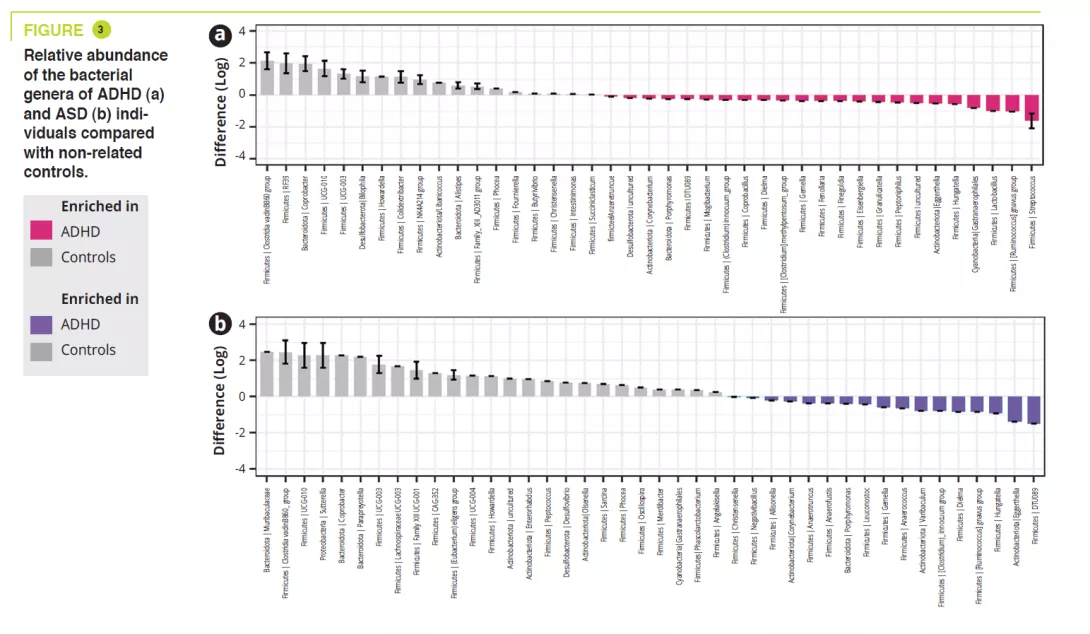
A total of 95 children aged 5-17 years, including 32 ADHD, 12 ASD, 11 ADHD/ ASD, 14, 5, 4 siblings, and 17 non-related controls. Gastrointestinal disorders were constipation: ADHD 15.6% (siblings 7.1%), ASD 8.3% (siblings 0%), ADHD/ ASD 18.2% (siblings 0%), controls 5.9%; abdominal pain: ADHD 3.1% (siblings 0%), ASD 16.7% (siblings 0%), ADHD/ ASD 18.2% (siblings 0%), controls 0%; and less frequently gastroœsophageal reflux. An atypical diet was found to be most common among the ASD children (50%), essentially a lack of variation in food intake. The analysis of the gut microbiota failed to find any variations in alpha-diversity between ADHD, ASD, ADHD/ASD and related or non-related controls; in contrast, it was significantly lower in siblings with ASD (figure 1). Gut microbiota composition was very similar between ADHD and ASD, as shown by beta-diversity, but this differed significantly between ADHD and ASD compared with non-related controls (figure 2). Analysis of the gut microbiota composition showed that some ADHD, ASD, or ADHD/ASD children had a relatively lower abundance of the Bacteroidetes phylum and greater abundance of Actinetobacteria. All groups were dominated by Bacteroides, Faecalibacterium, Blautia and Bifidobacterium genera; some children exhibited a high level of Prevotella. Differences in the abundance of bacterial genera were found between ADHD, ASD and controls (figure 3) but not between ADHD and ASD.
No difference was observed between the various groups, nor between related or non-related controls, for levels of faecal calprotectin nor for lipopolysaccharide-binding protein (LBP). However, there was no correlation either between faecal calprotectin and LBP with bacterial alpha- and beta-diversities. Measurement of various cytokines and chemokines failed to show significant differences between the various groups; however, several ADHD and ASD individuals had higher levels of IL1-RA compared with non-related controls and five ADHD children and one ASD child had concentrations of IFN- g higher than non-related controls. Lastly, weakly positive correlations were found between LBP and IL-8 (p=0.023), IL-12 (p=0.018), IL-13 (p=0.035) and PlGF (p=0.045), suggesting that disruption of the gut barrier function may result in immune dysregulation.
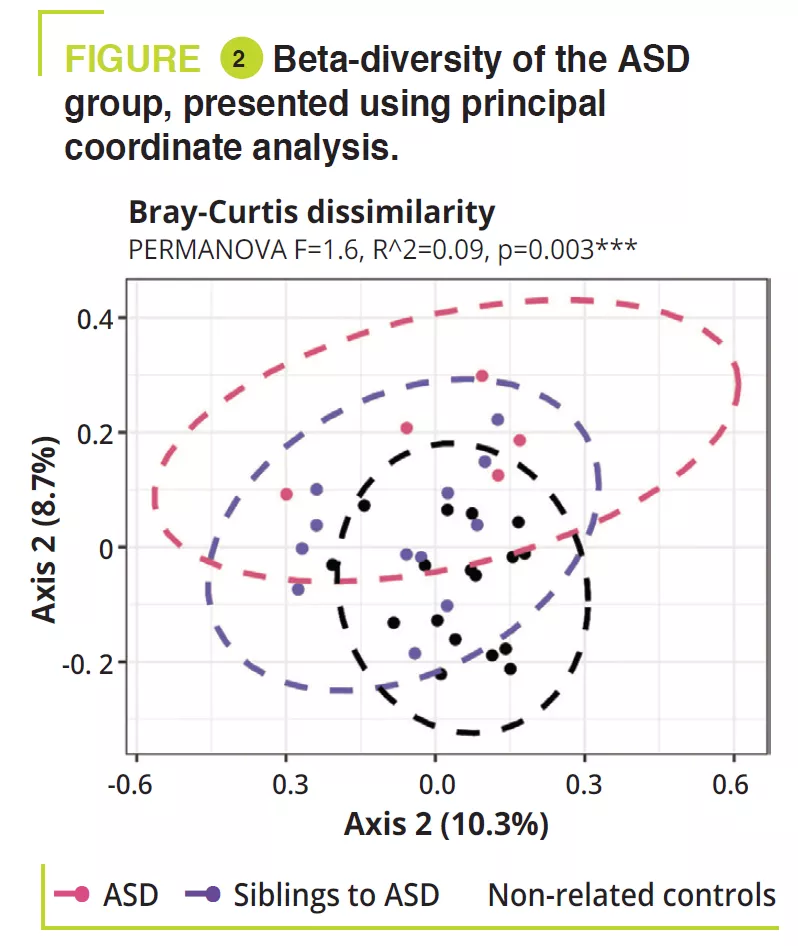
- There is confirmation of a modification to the gut microbiota in neurodevelopment disorders such as ADHD and ASD. The abnormal gut microbiota and the increase in intestinal permeability are probably involved in low-grade systemic inflammation
What are the consequences in practice?
This study was conducted on a small number of individuals, in particular concerning the small number of related controls. The gut microbiota, as well as intestinal permeability, may be relevant targets for the treatment of children and adolescents with ADHD and ASD.
CONCLUSION
ADHD and ASD children and adolescents have a similar gut microbiota, but one that is different from non-related controls. Moreover, variations in beta-diversity of the gut microbiota, as with an increase in LBP, were associated with differences between systemic pro and anti-inflammatory molecules.

