The gut microbiome as a determinant of healthy eating
By Anissa M. Armet 1 , João F. Mota 2,3 and Jens Walter 3
1 Department of Agricultural, Food & Nutritional Science, University of Alberta, Edmonton, Alberta, Canada
2 Faculty of Nutrition, Federal University of Goiás, Goiânia, Goiás, Brazil
3 APC Microbiome Ireland, School of Microbiology, Department of Medicine, and APC Microbiome Institute, University College Cork – National University of Ireland, Cork, Ireland
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Chronic non-communicable diseases (NCDs) have reached epidemic proportions in industrialized societies, a development clearly linked to changes towards western-style dietary patterns. NCDs are also linked to the gut microbiome, and research in animal models has established the causal importance of diet-microbiome interactions for the development of pathologies, as well as underlying mechanisms. Here we discuss what constitutes healthy eating from a microbiome science perspective and argue that a mechanistic understanding of diet-microbiome interactions can inform discussions of nutrition controversies and advance the development of healthier diets.
There is increasing evidence that the gut microbiome plays a significant role in influencing human health. Diet is central in this relationship, and western-style dietary patterns have played a major role in the recent exacerbation of chronic non-communicable diseases (NCDs) in socio-economically developed societies. Here, we discuss what constitutes healthy eating from a microbiome science perspective and apply this evidence to inform ongoing controversies in the nutrition field and development of microbiome-targeted nutritional strategies. We focus this article on
dietary recommendations in the general population with an aim of disease prevention, not patients with medical conditions who often have specific dietary requirements and should consult with a registered dietitian.
Healthy eating from a microbiome perspective
Whole plant foods versus processed foods
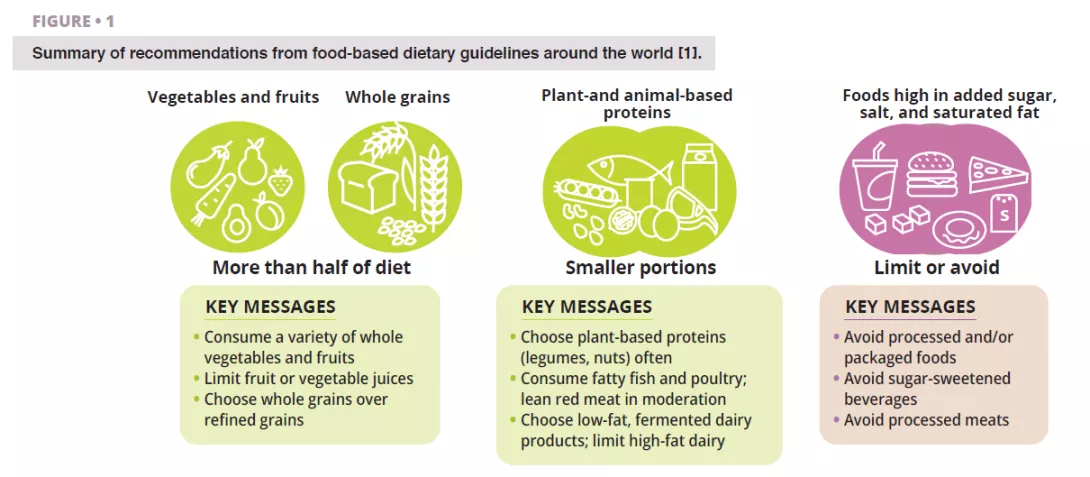
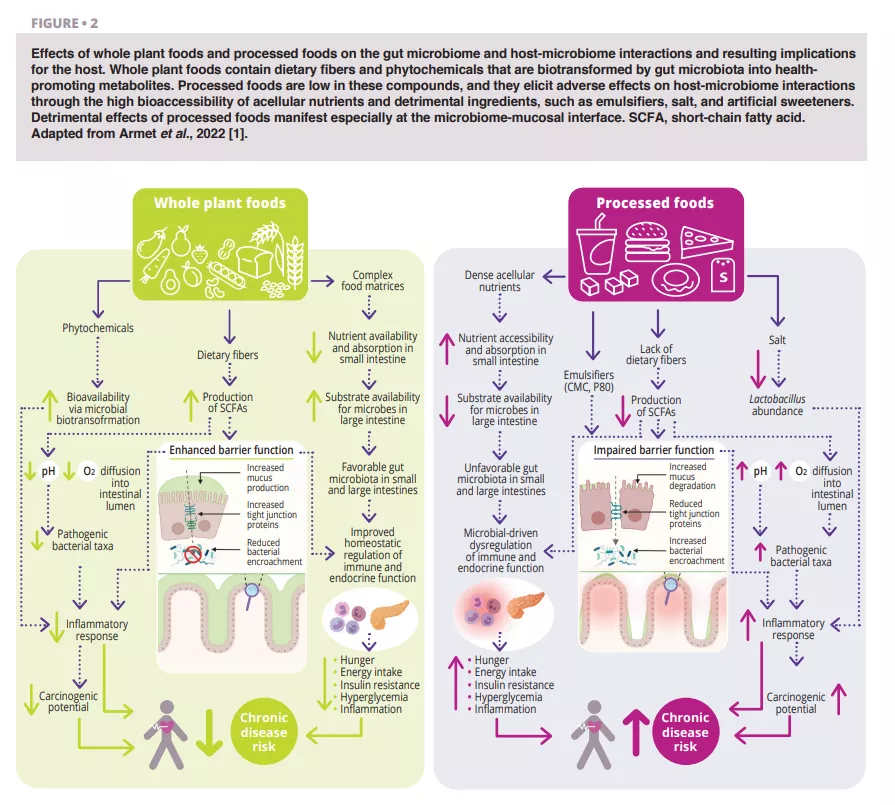
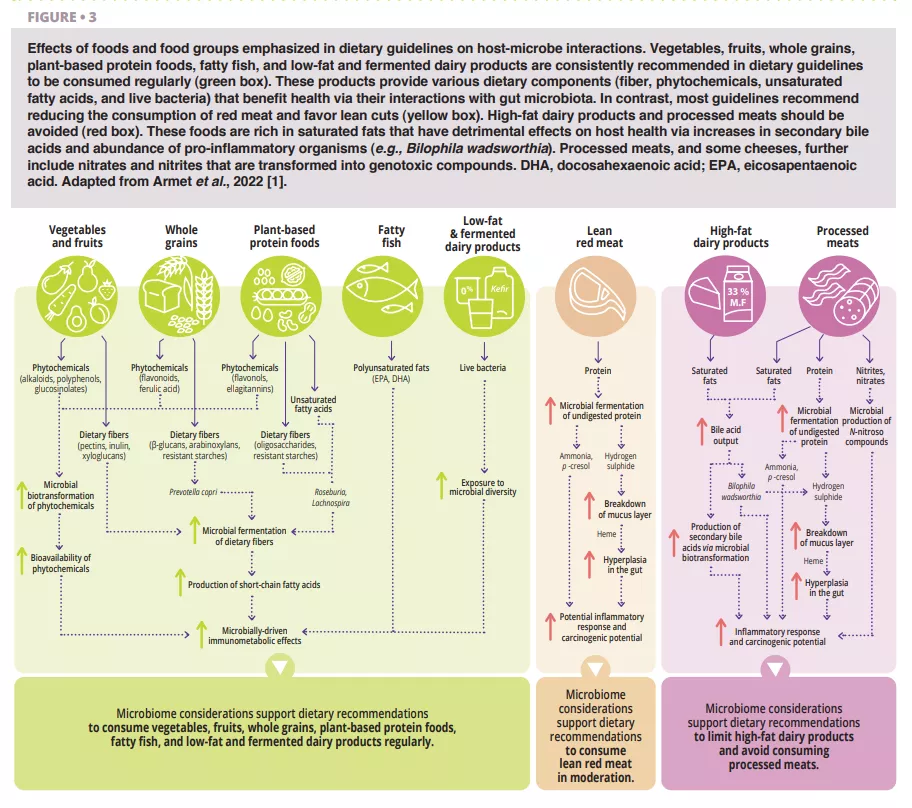
According to all dietary guidelines we reviewed [1], whole plant foods (vegetables, fruits, whole grains, legumes, and nuts) that have undergone limited processing should dominate a daily diet (figure 1). This recommendation is well-supported by a microbiome perspective (figure 2 and figure 3). Whole plant foods are the sole naturally occurring source of dietary fibers, some of which are fermentable and provide growth substrates for microbes. Plant variety may maintain microbiome diversity, and fiber fermentation results in metabolites such as short-chain fatty acids (SCFAs) that evoke a wide variety of metabolic (satiety-related hormones and improved insulin sensitivity), physiological (increased mucus production and tight junction expression), and ecological (pathogen inhibition) effects [2]. In addition, provision of substrates for bacteria prevents mucus degradation and downstream inflammation and infections in mice [3]. Phytochemicals present in whole plant foods, most of which are not absorbed in the small intestine, also undergo biotransformation by gut microbiota that increases their bioavailability, absorption, antioxidative, and immunomodulatory effects [4], but the significance of these interactions for health are less well-understood. Finally, the functional characteristics and nutritional quality (e.g., nutrient composition and accessibility) of most whole plant foods are vastly superior to processed foods, which often further contain food additives that impair microbiome composition and gut barrier function (figure 2).
Whole grains
The potential role of gut microbiome in the well-established metabolic and immunological benefits of whole grains is increasingly investigated. The bran layer of whole grains contains dietary fibers such as arabinoxylans and β-glucans that are fermented by gut microbiota into beneficial metabolites. The anti-inflammatory effects of whole grains have been linked to enrichment of SCFA-producers [5]. In ex-germ-free mice colonized with fecal microbiota from humans that responded to whole grain barley kernel bread and contained Prevotella, it was observed improvement in glucose tolerance that mirrored effect in humans [6]. In addition, individuals with excess body weight that harbor Prevotella at baseline show elevated weight loss on a whole grain-rich diet [7]. These studies suggest that at least some of the metabolic benefits of whole grains are mediated by the gut microbiome (figure 3).

Sources of protein
Most dietary guidelines recommend consuming plant-based protein foods (e.g., legumes, nuts), and fish (e.g., fatty fish) and poultry at the expense of other animal sources of protein, specifically red meat (figure 1). Legumes and nuts are rich in fibre and contain phytochemicals and omega-3 fatty acids that influence host-microbe interactions (figure 3). Daily supplementation of walnuts and almonds increased relative abundances butyrate- producers, specifically Roseburia [8]. Mung bean supplementation reduced weight gain and fat accumulation in mice fed high-fat diets but not in germ-free mice fed the same diets, establishing a causal role of the microbiome [9]. Among all animal- based protein foods, fatty fish is likely to display the largest microbiome-driven immunological and metabolic benefits [1].
Dietary patterns
The realisation that health is not primarily influenced by individual foods or nutrients but their interconnectedness and synergistic effects led to an emphasis on dietary patterns in several recently updated dietary guidelines, such as the 2020-2025 Dietary Guidelines for Americans and Canada’s food guide. Mediterranean diet combines many of the food groups that have favorable effects on host-microbe interactions. Several randomized-controlled trials were conducted to investigate these interactions, and showed that metabolic, immunological, and cognitive benefits of
a Mediterranean diet were linked to increases in Faecalibacterium prausnitzii and Roseburia abundances [10].
How does the gut microbiome inform controversies around healthy eating?
Red and processed meats
Most dietary guidelines and several medical societies recommend the reduction of red meat and the avoidance of processed meats, although a series of systematic reviews from 2019 concluded that there is only weak evidence of their links to adverse health outcomes [11]. The gut microbiome provides a helpful perspective in this controversy. Proteolytic fermentation of meat protein by gut microbes increases toxic metabolites such as ammonia, p-cresol, and hydrogen sulphide [12]. Being high in saturated fat, processed meats further stimulate secretion of bile acids into the small intestine that are then transformed into secondary bile acids by microbes. In addition, the curing agents used in processed meats, nitrate and nitrite, are substrates for microbial biotransformation to N-nitroso compounds. Toxicological considerations therefore support current dietary recommendations (figure 3).
The metabolites resulting from protein fermentation (e.g., hydrogen sulphide, ammonia) are of lower toxicity and not currently classified as human carcinogens, supporting the conclusion that moderate consumption of lean red meat is likely of limited risk. In contrast, the N-nitroso compounds and secondary bile acids that result from consumption of processed meats are carcinogenic, supporting recommendations to avoid or minimize the consumption of processed meat.

Microbiome centric recommendations for a healthy diet
- Follow recommendations in dietary guidelines (figure 1).
- Maximize diversity in plant sources consumed and try to push fiber levels beyond what is currently recommended (25-38 grams/day).
- Minimize processed foods with high amounts of added sugar, salt, saturated and trans fats as well as processed meats and high-fat dairy.
- Include fermented foods with live microbes (no heat treatment) that are low in sugar, fat, and salt such as yoghurt, fermented vegetables, kefir, and kombucha.
Dairy
Most dietary guidelines recommend skimmed and low-fat (0-2%) dairy products and suggest avoiding high-fat (> 25%) dairy products (e.g., certain cheeses, cream-based products, butter). However, there is no consensus on full-fat dairy (~ 3.5%), which is discouraged in some dietary guidelines, although its detrimental effects have been questioned. Interactions between dairy fat and the gut microbiome are relevant for this discussion. Milk-derived saturated fats induce Bilophila wadsworthia, which is pro-inflammatory and caused diseases such as colitis [13] in mouse models. These mechanistic findings support dietary recommendations to limit dairy to low-fat varieties (figure 3).
Low-carbohydrate diets
Low-carbohydrate diets are popular as they can achieve remarkable short-term weight loss and metabolic benefits, although results may not be sustainable in the long-term. These diets are high in fat and/or protein and often low in fiber.
Consequently, they result in a detrimental metabolic profile with increased concentrations of N-nitroso compounds and decreased levels of butyrate and anti-inflammatory phenolic compounds [14]. Due to their effects on gut microbiota, low-carbohydrate diets might therefore be detrimental to health when consumed over longer periods.

Advancing healthy eating through the microbiome
Although international dietary guidelines are highly consistent and provide excellent guidance on what constitutes healthy eating, there is scope for improvements and innovations through a more systematic consideration of the microbiome.
Evolutionary considerations and microbiome restoration
The human-microbiome symbiosis has evolved over millions of years in a very different environmental and nutritional context. Industrialization, which led to a substantial increase in NCDs, depleted microbiome diversity, diminished enzymatic capacity of the microbiome for carbohydrate utilization, enriched for mucus- degrading organisms and enzymes, and led to a loss of microbial symbionts. There is therefore an evolutionary-based argument to boost fiber intake beyond the 25-38 grams per day that are currently recommended in dietary guidelines, and this is supported by both observational and intervention studies [15]. In addition to promoting increased fibre intake from whole foods, there is strong scientific rationale to redress the impact of industrialization on the gut microbiome through prebiotic, probiotic, and synbiotic strategies. Products that attempt to restore and diversify the microbiome are already on the market, but research on their clinical benefits is in its infancy (see below).
Probiotics and prebiotics
Although many studies have shown clinical benefits when probiotics and prebiotics are used for specific medical targets, few health claims have been approved by regulatory agencies. In addition, there is little evidence that their
consumption reduces the risk of NCDs, and the vast majority of national dietary guidelines have not made recommendations to include them as part of a healthy diet. There is great potential to develop probiotics, prebiotics, and their combination (synbiotics) to prevent chronic disease more systematically. Ongoing research explores the use of these strategies to redress the impact of industrialization on gut microbiome diversity and function. Products have been developed and marketed in this area, but await clinical validation in well-controlled RCTs, and the available research is too preliminary to make any general recommendations.
Live microbes
Another hallmark of industrialization is reduced microbial exposure. The biodiversity hypothesis states that contact with natural environments is required to enrich the human microbiome, promote immune balance, and protect from allergy and inflammatory disorders. Probiotics provide
live microbes and have been studied and marketed in this context for decades (see box “Probiotics and prebiotics”). In addition, fermented foods, such as kefir, yoghurt, kombucha, and Sauerkraut can, if consumed raw, contain high numbers of live microbes (bacteria and fungi). Although
microbes present in fermented foods do not colonize the human gut due to their non-native nature in the human gut ecosystem, they are still detectable within human fecal microbiota and might interact directly with the host.

Some dietary guidelines include fermented foods, such as yogurt and fermented milks, as part of their recommendations, and their benefits are increasingly reported in observational studies and smaller RCTs, but more well controlled human trials are needed.
Precision nutrition
Humans differ in their response to dietary interventions, which questions the onesize- fits-all approach currently applied in dietary guidelines. Precision or personalized nutrition aims to tailor nutritional recommendations to an individual’s biology (genes, metabolism, etc.). Microbiome measurements might become a key component of precision nutrition strategies. Although several companies already offer personalized dietary advice based on the fecal microbiome, these services are not validated by any regulatory authority, leaving uncertainty regarding the accuracy of the recommendations. National dietary guidelines currently do not consider precision or personalized approaches, and their implementation will be challenging on a population scale. Although there is a scientific rationale to personalize nutrition, it is important to emphasize that most individuals will benefit from the dietary recommendations discussed above.
Conclusion
The gut microbiome may constitute the “black box” of nutrition research as many physiological effects of diet may be influenced by diet-microbehost interactions. Additional research is needed to determine to what extent the microbiome makes causal contributions to physiological effects
of diet and which mechanisms detected in animal models apply to humans. Nevertheless, the available evidence is strongly in support of an important role of the gut microbiome in the effects of diet, emphasizing that a mechanistic understanding of diet-microbiome interactions can inform
nutrition controversies and advance the development of healthier diets.
1. Armet AM, Deehan EC, O’Sullivan AF, et al. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe 2022; 30: 764-85.
2. Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes 2020; 11: 411-55.
3. Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016; 167: 1339-53 e21.
4. Chang SK, Alasalvar C, Shahidi F. Superfruits: phytochemicals, antioxidant efficacies, and health effects - a comprehensive review. Crit Rev Food Sci Nutr 2019; 59: 1580-604.
5. Martínez I, Lattimer JM, Hubach KL, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 2013; 7: 269-80.
6. Kovatcheva-Datchary P, Nilsson A, Akrami R, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 2015; 22: 971-82.
7. Roager HM, Christensen LH. Personal diet-microbiota interactions and weight loss. Proc Nutr Soc 2022: 1-28.
8. Creedon AC, Hung ES, Berry SE, Whelan K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients 2020; 12: 2347.
9. Nakatani A, Li X, Miyamoto J, et al. Dietary mung bean protein reduces high-fat diet-induced weight gain by modulating host bile acid metabolism in a gut microbiota-dependent manner. Biochem Biophys Res Commun 2018; 501: 955-61.
10. Kimble R, Gouinguenet P, Ashor A, et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: a systematic review of randomized controlled trials and observational studies. Crit Rev Food Sci Nutr 2023; 63: 8698-719.
11. Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the Nutritional Recommendations (NutriRECS) Consortium. Ann Intern Med 2019; 171: 756-64.
12. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014; 12: 661-72.
13. Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012; 487: 104-8.
14. Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 2011; 93: 1062-72.
15. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019; 393: 434-45.

