Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives of patients
COMMENTED ARTICLE - ADULTS’ SECTION
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Author
Commentary on the article by Raygoza Garay et al. Gastroenterology 2023 [1]
Background and aims: The cause of Crohn’s disease (CD) is unknown, but the current hypothesis is that microbial or environmental factors induce gut inflammation in genetically susceptible individuals, leading to chronic intestinal inflammation. Case-control studies of patients with CD have catalogued alterations in gut microbiome composition; however, these studies fail to distinguish whether gut microbiome composition alteration is associated with initiation of CD or is the result of inflammation or a drug treatment. Methods: In this prospective cohort study, 3,483 healthy first-degree relatives of patients with CD were recruited to identify the gut microbiome composition that precedes the onset of the disease and to what extent this composition predicts the risk of developing CD. A machine learning approach was applied to the analysis of gut microbiome composition (based on 16S ribosomal RNA sequencing) to define a microbial signature that associates with future development of CD. The performance of the model was assessed in an independent validation cohort. Conclusion: This study is the first to demonstrate that gut microbiome composition is associated with future onset of CD and suggests that the gut microbiome contributes to the pathogenesis of Crohn’s disease.
What do we already know about this subject?
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterised by recurrent chronic inflammation of the intestine. The cause of CD is unknown, but the current hypothesis is that microbial or environmental factors induce inflammation of the gut in genetically predisposed individuals, leading to inflammation and chronic lesions. Case-control studies of patients with CD have catalogued alterations in the gut microbiome composition [1]. However, these studies fail to distinguish whether the altered gut microbiome composition is associated with the onset of Crohn’s disease or is the result of inflammation or a drug treatment. To answer these questions, the Canadian project GEM (Genetic Environmental Microbial), a prospective cohort study in healthy first-degree relatives of individuals with Crohn’s disease, was designed to identify the parameters associated with the development of Crohn’s disease. Among parameters, the authors studied the gut microbiome profile preceding the onset of CD and to what extent this composition predicts the risk of developing CD. The authors applied a machine learning approach to the analysis of the composition of the gut microbiome in a large cohort of healthy first-degree relatives of individuals with Crohn’s disease (N = 3,483) with the aim of defining a microbial signature associated with the risk of developing CD.
What are the main insights from this study?
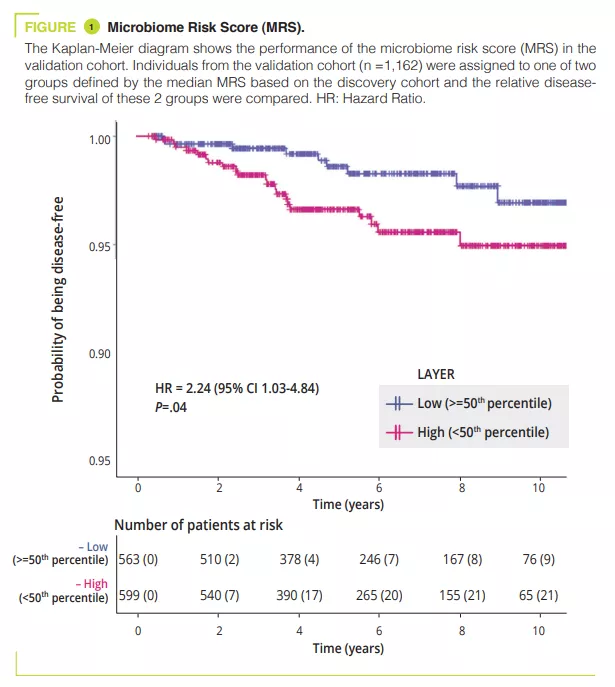
On the basis of data from the GEM cohort, the authors developed and validated a microbiome risk score (MRS) used to classify individuals who will develop CD in the future. The taxa contributing most to the MRS were an increased abundance of Ruminoccus torques and Blautia which was positively correlated to the MRS (suggesting a harmful effect of these taxa), whereas the abundance of the genus Roseburia was negatively correlated with the MRS (suggesting a protective effect of this genus). Lastly the authors observed than an increase in the abundance of the genus Faecalibacterium was inversely related to an increase in the MRS. This study is the first to demonstrate that a decrease in the abundance of Faecalibacterium may be a preclinical signature of CD which may be observed many years before the onset of the disease, suggesting a causal relationship with the decrease in this anti-inflammatory bacteria [2] . Significantly, the changes in the microbiome preceding the onset of CD were observed independently of the existence of intestinal inflammation (indicated by faecal calprotectin levels).
The authors also performed a metabolomic analysis of stool samples from a sub-group of the cohort. Cytosine and its derivative, cytidine showed the strongest negative correlation with the MRS.
Moreover, the pre-CD signature of the MRS was associated with a reduction in metabolites having anti-inflammatory or antioxidant activity such as gentisate and nicotinate. These protective metabolites were also positively correlated with the abundance of Faecalibacterium and Lachnospira, which indicates a potential biological interaction between the abundance of these metabolites and the microbial composition.
- The gut microbiota becomes altered several years before the diagnosis of Crohn’s disease, independently of the existence of intestinal inflammation, suggesting that the microbiome has a causal role in Crohn’s disease
- A Microbiome Risk Score may identify subjects at most risk of developing Crohn’s disease
- Early action targeting the microbiome may be proposed in patients identified as being at risk of developing Crohn’s disease
What are the consequences in practice?
This study suggests that the analysis of the microbiome of healthy individuals at risk of developing Crohn’s disease may identify the most high risk subjects and thus permit their close supervision and the possibility of initiating interventions designed to modify the microbial imbalance and therefore reduce the risk of developing the disease.
CONCLUSION
This study is the first to demonstrate that changes in gut microbiome composition occur many years before the diagnosis of Crohn’s disease. This suggests that the gut microbiome contributes to the pathogenesis of Crohn’s disease and that it may be a potential target for prevention and/or therapy.
1. Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic. Approaches. Gastroenterology 2017; 152: 327-39.e4.
2. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 2008; 105: 16731-6.


