FMT, in practice: from the donor to the side effects
We can easily imagine how skin, kidney or lung transplants are performed. But what about fecal microbiota transplant (FMT)? Faced with the explosion of new research on FMT, health authorities from several countries (including France) published recommendations aiming at regulating this practice, and especially donor selection, because a transplant requires a donor, and not anyone is eligible to donate their stool!
- Learn all about microbiota
- Microbiota and related conditions
- Act on your microbiota
- Publications
- About the Institute
Healthcare professionals section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Is fecal microbiota a drug?
The answer depends on the country. In France and in the US, fecal microbiota is considered as a drug. It is not the case in the UK, Denmark or the Netherlands. In France, the National Agency for the Safety of Medicine and Health Products (ANSM) published in March 2014 and updated in 2016 a document regulating FMT which describes the procedure, especially the donor selection process.
Specific procedure
In France, FMT must be prepared under the responsibility of the in-house pharmacy of a health facility3. The collected stools are diluted, mixed, filtered and then filled into syringes before being administered. They can also be frozen, which provides the opportunity to create stool banks that are available at any moment5. The ANSM adds that “freezing could also limit the risk of transmission of infectious agents and bypass the pre-screening step (the screening could then be carried out on the transplant itself”.
VERY LIMITED ADVERSE EVENTS
Adverse events5 of FMT are usually moderate and, for the most part, of gastrointestinal nature. They occur within hours following the transplant and disappear within 48 hours:
- diarrhea in 75% of patients,
- abdominal pain in 50% of
them,
- more rarely, constipation.
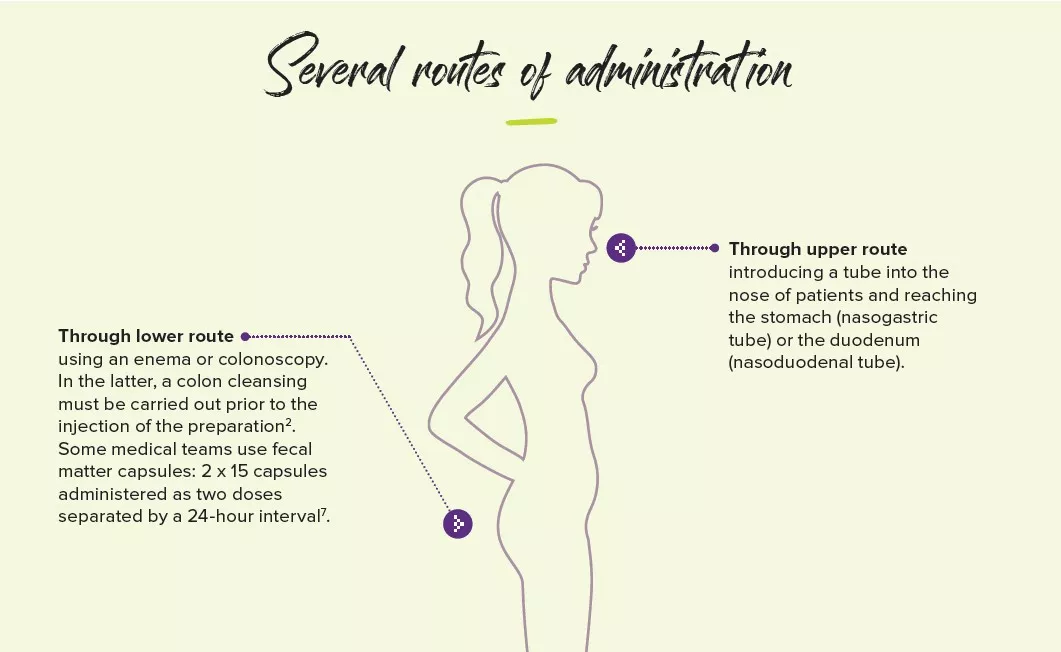
Severe adverse effects are extremely rare, but they are enough to warrant a strict donor selection process: bacteremia, norovirus infection (two published cases), increased weight (one reported case), acute pulmonary edema (one reported case). Some are related to the administration route, for instance gastrointestinal perforations
6 https://www.snfge.org/content/la-transplantation-de-microbiote-fecal-tmf-da













