Microbiota in Covid-19 pandemic
Overview
By Pr. Conceição Calhau
NOVA Medical School, New University of Lisbon, Portugal
By Pr. Pedro Povoa
NOVA Medical School, New University of Lisbon, Portugal; Polyvalent Intensive Care Unit, Hospital São Francisco Xavier, CHLO, Lisbon, Portugal; Center for Clinical Epidemiology, OUH Odense, University Hospital, Denmark
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
For the first time, the gut microbiota diversity is pointed out as a prognostic biomarker of Covid-19 severity. Thus, the microbiota changes as reliable biomarkers in the context of Covid-19 represent a key piece of the disease puzzle, thus highlighting the clinical priority for prevention and possibly new therapeutic strategies. In 2020, the new coronavirus severely affected certain groups of the population, more specifically, the elderly and people with obesity, hypertension, diabetes [1]. Interestingly, publications showed that dysbiosis is a common factor in all these patients [2, 3].
GUT MICROBIOTA SIGNATURES IN COVID-19 PATIENTS
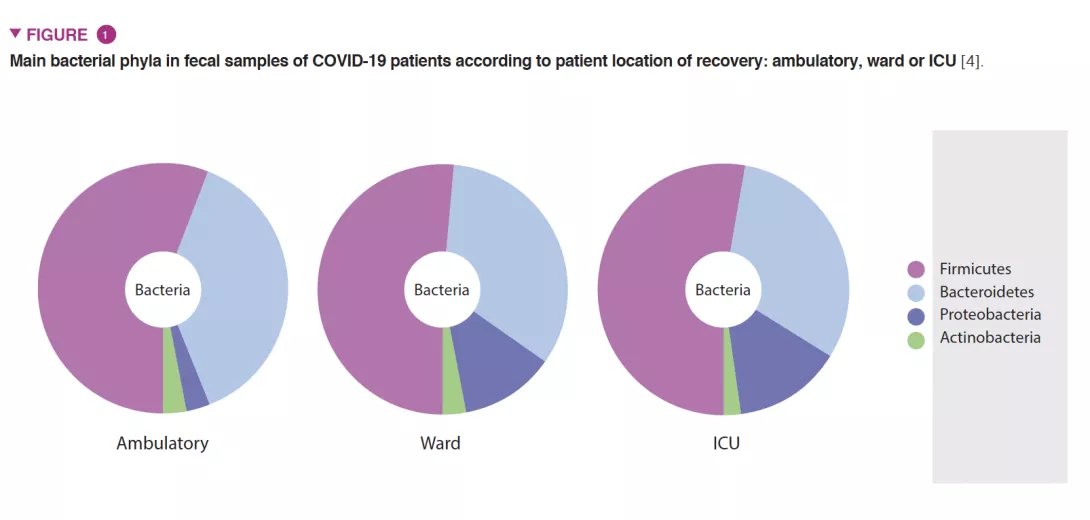
As gut microbiota has been considered a hot topic in the scientific community with a pivotal role on host immune and inflammatory functions, we investigated if changes in gut microbiota composition are associated with an increased clinical severity of Covid-19 [4]. A national multicenter cross-sectional study was conducted in 115 Covid-19 patients categorized by: 1) location of recovery from Covid-19: ambulatory (household isolation), ward or intensive care unit; and 2) Covid-19 severity scale: asymptomatic/mild-to-moderate or severe. Severely ill patients exhibited profound changes in gut microbiota composition compared with mild-to-moderate patients in ambulatory or admitted to the hospital ward (Figure 1). These changes included: 1) lower overall gut microbial diversity; 2) lower abundance of beneficial butyrate-producing bacteria such as Roseburia and Lachnospira; 3) lower Firmicutes/ Bacteroidetes ratio; 4) higher abundance of Proteobacteria. In addition, we detected the virus in fecal samples, which must be considered in public health recommendations [5, 6] Publications from other colleagues have shown that low diversity could be a clinical biomarker predicting greater risk of severity [7-9].

RESPIRATORY MICROBIOTA IN COVID-19 CRITICALLY ILL PATIENTS
The conventional wisdom was that the healthy lungs were sterile. In the last decade, the application of techniques of microbiota research clearly showed that was not the case. The lungs are colonized by a very low bacterial load compared with the gut [10]. The different parts of the respiratory tract (oropharynx, airway, lungs) present different diversity and compositions related to the colonization sources, colonization rates, extinction rates and distances between each other according to the adapted island model, being the “mainland” the oral cavity [11]. Recent studies in severe Covid-19 patients showed dysbiosis of the airway microbiota (analyzed in BALF samples) similar to the dysbiosis observed during lower respiratory tract infections, pneumonia for example [12, 13]. In addition, Acinetobacter – a common gram-negative non-fermenting bacilli pathogen of ventilator-associated pneumonia that is the most severe ICU-acquired infection in patients undergoing invasive mechanical ventilation – was a common bacterial genus found in lung tissues in deceased patients [14]. The presence of some pathogens in the lung of deceased patients and in the oral cavity is related to the adapted island model migration [15]. As a result of the Covid- 19 associated immune dysregulation, several epidemiologic studies showed an increased risk of hospital acquired infections, namely ventilator associated pneumonia as our group showed. In our study we found that Covid-19 patients presented twice the risk of ventilator associated pneumonia in comparison to non-Covid-19 patients [16].
Conclusion
Microbiota and Covid-19 studies may open perspectives for the development of therapeutic interventions (probiotics, prebiotics…) that aim to correct dysbiosis observed in severe Covid-19 patients. These interventions are expected to increase overall bacterial diversity and the abundance of commensal bacteria, thereby contributing to inhibit the overgrowth of opportunistic pathogens. These studies could also have implications in the design of effective Covid-19 vaccines, as one factor known to control vaccine efficacy could be the gut microbiota.






