Early-life formula feeding is associated with gut microbiota alterations and increased antibiotic resistance in infants
COMMENTED ARTICLE - CHILDREN’S SECTION
By Pr. Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Comments on the article by Pärnänen KMM et al. Am J Clin Nutr 2021 [1]
Infants are at a high risk of acquiring fatal infections whose treatment relies on functioning antibiotics. Antibiotic resistance genes (ARGs) are present in high numbers in the gut microbiomes of antibiotic-naive infants, and infant mortality caused by resistant infections is high.
In this article the authors aim to determine the impact of early exposure to formula on the ARG load in neonates and infants born either pre- or full-term. One assumption was that diet exerts selective pressure which influences the microbial community in the gut of infants and formula exposure increases the abundance of taxa-carrying ARGs.
The study showed that formula-fed infants had a higher relative abundance of opportunistic pathogens such as Staphylococcus aureus, S. epidermidis, Klebsiella pneumoniae, K. oxytoca and Clostridioides difficile. Formula-fed infants also had significantly fewer typical infant bacteria such as bifidobacteria, which have potential health benefits.
The novel finding of a correlation between formula exposure and higher neonatal ARG burden shows that clinicians should consider feeding mode in addition to antibiotic use in the first months of life to minimise the proliferation of antibioticresistant gut bacteria in infants.
WHAT DO WE ALREADY KNOW ABOUT THIS SUBJECT?
Antibiotic-resistant bacteria are the cause of many neonatal deaths. The emergence of resistant bacteria is favoured by the use of antibiotics, which is associated with a higher number of antibiotic-resistance genes (ARGs) carried by these resistant or multi-resistant bacterial strains. Mobile genetic elements (MGEs) transmit ARGs between bacteria. It is also known that the type of diet modifies the intestinal microbiota, as well as the quantity of ARGs. The magnitude of the impact of infant diet on resistance has not been adequately described in the literature.
WHAT ARE THE MAIN INSIGHTS FROM THIS STUDY?
The authors included 46 infants born prematurely between 26 and 37 weeks of gestation of which 21 were fed with formula, 20 with fortified breast milk and 5 with breast milk. Stools were collected within 36 days to analyse the composition of gut microbiota and presence of ARGs. Thirty infants received an antibiotic treatment: stools were collected approximately two weeks after the end of treatment to limit confounding effects.
To compare the results with literature data, a meta-analysis of five studies including 696 neonates with similar data was analysed in parallel.
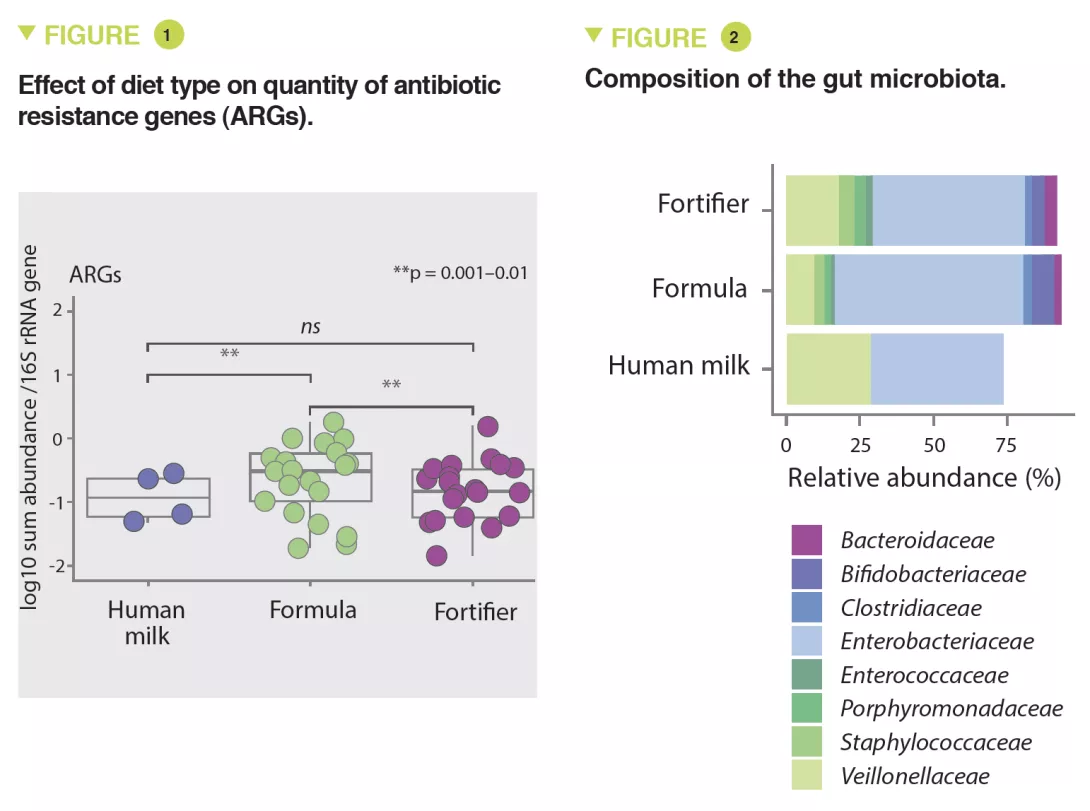
The results showed that infants fed with formula had significantly higher amounts of ARGs than infants fed with fortified breast milk (x 3.6; 95% CI, 1.61-8.9) or breast milk (x 4.3; 95% CI, 1.61-11.56) (p < 0.01) (Figure 1). The abundance of MGEs was similarly increased (p < 0.05).
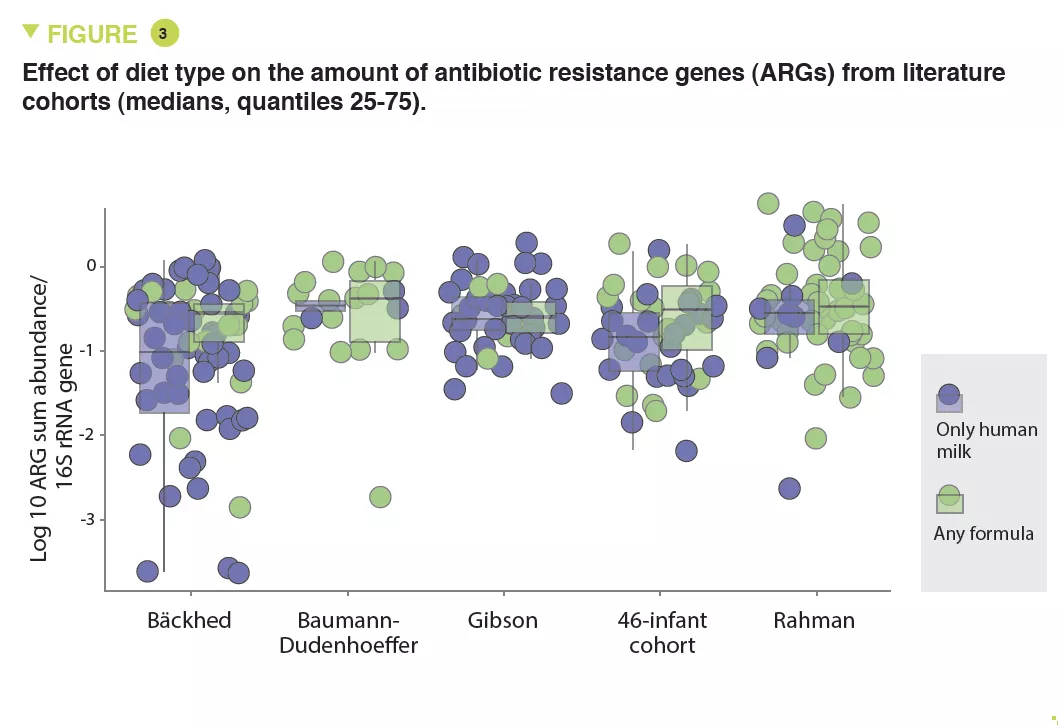
The abundance of Enterobacteriaceae, whose genome is known to contain more mobile ARGs, was higher in formula-fed infants (p < 0.05) (Figure 2) and tended to be inversely correlated to gestational age (p < 0.1). It was noted that the longer the gestation the lower the abundance of these ARGs (x 0.72; 95% CI, 0.57-0.89) (p < 0.001). Several ARGs were significantly more abundant in formula-fed infants including genes encoding the extended-spectrum beta-lactamase present in Klebsiella (p < 0.05). Similar results were observed in the meta-analysis with a 70% relative increase in ARGs in formula-fed neonates (p = 0.013). The median ARG load was higher in the formula-fed infants in all cohorts (Figure 3). Finally, an analysis of gut microbiota revealed that bacteria belonging to Bifidobacteriaceae, Veillonellaceae, Clostridiaceae, Lachnospiraceae, and Porphyromonadaceae families (including strict anaerobe bacteria) were depleted in neonates fed with infant formula; conversely, facultative anaerobe bacteria belonging to the Enterobacteriaceae, Staphyloccoccaceae, and Enterococcaceae families were increased (p < 0.05).
Similarly, several potentially pathogenic species including facultative anaerobe species such as S. aureus, S. epidermidis, K. pneumoniae, K. oxytoca, and a strict anaerobe species Clostridioides difficile, were enriched in formula-fed neonates (p < 0.001). Thus, the use of infant formulas enhances the proliferation of pathogenic bacteria with ARGs.
WHAT ARE THE CONSEQUENCES IN PRACTICE?
These results support the benefit of breastfeeding. Feeding premature neonates with infant formula is associated with a 70% increase in ARGs compared to those fed exclusively with breast milk. Enriched breast milk results in a smaller increase in these ARGs.
KEY POINTS
- Use of infant formula is associated with increased antibiotic resistance genes (ARGs).
- This resistance is transmitted between bacteria by mobile genetic elements, with a predominance of Enterobacteriaceae in formula-fed infants.
Conclusion
In addition to the appropriate use of antibiotics, it is important to consider the type of diet for premature neonates, with a preference for breast milk to avoid the proliferation of resistant bacteria.

