A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumours
COMMENTED ARTICLE - ADULTS’ SECTION
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Author
Comment on the article by Dohlman et al. (Cell 2022 [1])
Fungal microorganisms (mycobiota) comprise a small but immunoreactive component of the human microbiome, yet little is known about their role in human cancers. Pan-cancer analysis of multiple body sites revealed tumour-associated mycobiomes at up to 1 fungal cell per 104 tumour cells. In lung cancer, Blastomyces was associated with tumour tissues. In stomach cancers, high rates of Candida were linked to the expression of proinflammatory immune pathways, while in colon cancers Candida was predictive of metastatic disease and attenuated cellular adhesions. Across multiple GI sites, several Candida species were enriched in tumour samples and tumour-associated Candida DNA was predictive of decreased survival. The presence of Candida in human GI tumours was confirmed by external ITS sequencing of tumour samples and by culture-dependent analysis in an independent cohort. These data implicate the mycobiota in the pathogenesis of GI cancers and suggest that tumour-associated fungal DNA may serve as diagnostic or prognostic biomarkers.
What do we already know about this subject?
Cancer is one of the leading causes of death worldwide. The tumorigenesis, progression and treatment-response of cancer are influenced by various interactions between the immune system of the host and bacteria in the microbiota. However, the role of fungi (mycobiota) in these processes remains largely unexplored. Fungi and bacteria co-colonise the gastrointestinal tract, skin epithelium, airways and reproductive organs of mammals, forming a complex ecosystem of microbe-microbe and host-microbe interactions with significant implications for human health. While fungal infections account for over 1.5 million deaths worldwide each year, they only account for 0.1% of microbial DNA in the gut, suggesting a disproportionate influence of species from this kingdom on the overall gut microbiome and host immunity. Whether referring to viruses, bacteria or fungi, an ever growing body of scientific evidence suggests a link between the human microbiome and cancer and its outcomes. Several cases showing the association between bacterial species and cancer development/progression have been observed in recent years. Helicobacter pylori is responsible for approximately 75% of the risk attributable to gastric cancer, while genotoxic Escherichia coli, Bacteroides fragilis, Streptococcus bovis/gallolyticus and Fusobacterium nucleatum have been implicated in colorectal carcinogenesis [2]. The common feature of these bacteria is their ability to trigger chronic inflammation, a feature considered to contribute to their tumorigenic capacity. Recent reports have also identified intracellular bacteria in many types of tumour [3].
The mycobiome plays a key role in the activation of innate immunity in the gut. Mycotoxins and bioactive amines have been associated with carcinogenesis. Recent experimental studies support fungal involvement in cancer in some contexts [4]. Sequencing data from tumour banks have revealed the presence of microbial sequences, although the fungal component has not yet been explored.
What are the main insights from this study?
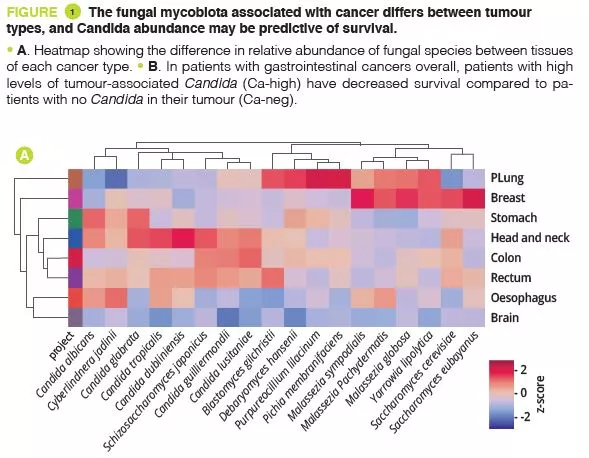
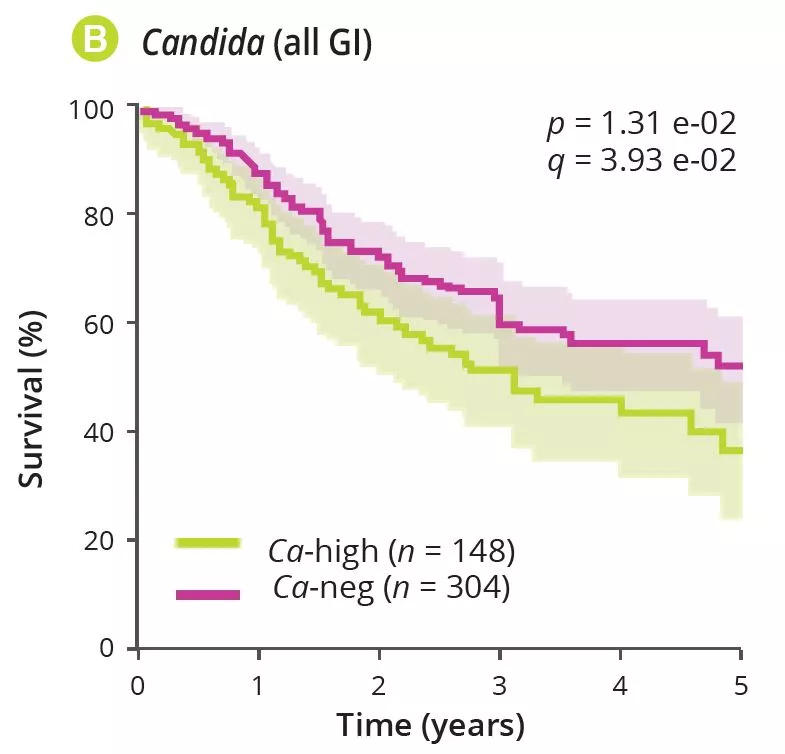
By analysing several types of cancer using “The Cancer Genome Atlas” (TCGA), the authors extracted tumour-associated mycobiome profiles with a species-level resolution. After eliminating contamination and false-positive signals, the authors reported that fungal compositions varied according to the type of cancer, and that some fungi were tumour-type specific, in both gastrointestinal and non-gastrointestinal locations (figure 1A). Overall, up to one fungal cell per 104 human tumour cells were observed, a rate consistent with the finding that fungi make up 0.1-1% of the microbiome, while bacteria are estimated to make up less than 1% of tumour cells [2, 3]. Abundant numbers of several species of Candida, Saccharomyces cerevisiae and Cyberlindnera jadinii have been found in gastrointestinal tumours, while Blastomyces and Malassezia species are abundant in lung and breast tumours, respectively. The authors then showed that several Candida species are alive and transcriptionally active in the tumour. Finally, the abundance of some fungi within the tumour could predict host tumour gene expression, disease status and survival (figure 1B), although these findings still need to be confirmed. Overall, these results suggest an involvement of fungi, especially Candida, in the pathogenesis of gastrointestinal cancers but also highlight their potential as a therapeutic target and prognostic tool.
KEY POINTS
- A pan-cancer analysis of the mycobiome revealed the presence of fungi within tumour tissue
- Gastrointestinal tumours contain live and transcriptionally active Candida
- Abundant Candida DNA is found in some tumour tissue, and could be indicative of poor prognosis
What are the consequences in practice?
Alongside bacteria, this study reported the presence of fungi in many gastrointestinal and non-gastrointestinal tumours, with some degree of specificity across tumour types and a potential for predicting severity. These results suggest that fungi play a role in the cancer process and its severity. They could also pave the way for the development of new biomarkers or new cancer treatments targeting the fungal component.
CONCLUSION
An analysis of multiple gastrointestinal and non-gastrointestinal tumours identified tumour-associated fungi, especially Candida enrichment in gastrointestinal cancers. Fungi may also play a role in carcinogenesis. Tumourassociated fungal DNA could serve as a prognostic marker in this context and fungi could represent a new therapeutic target in cancer.

"Interesting developments. Thank you for caring about humanity." -sturehp (From Biocodex Microbiota Institute on X)
"Tumour-associated fungal DNA may serve as diagnostic or prognostic biomarkers. Very interesting. I am curious to see what comes out of this." -Just me. (From Biocodex Microbiota Institute on X)
1. Dohlman AB, Klug J, Mesko M, et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022 ; 185 : 3807-22.e12.
2. Sepich-Poore GD, Zitvogel L, Straussman R, et al. The microbiome and human cancer. Science 2021 ; 371 : eabc4552.
3. Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020 ; 368 : 973-80.
4. Alam A, Levanduski E, Denz P, et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 2022 ; 40 : 153-67.e11.







