The maternal microbiome promotes placental development in mice
COMMENTED ARTICLE - Adults’ section
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Comments on the article by Pronovost et al. (Science Advances 2023) [1]
The maternal microbiome is an important regulator of gestational health but how it affects the placenta as an interface between mother and foetus has not yet been researched. The authors of this article show that maternal gut microbiota promotes placental development in mice. Depletion of the maternal gut microbiota limits placental growth and impairs foeto-placental vascularisation. The maternal gut microbiota modulates metabolites in maternal and foetal circulation. Short-chain fatty acids (SCFAs) stimulate tube formation in cultured endothelial cells and prevent placental vascularisation abnormalities in microbiota-deficient mice. In addition, in a maternal-malnutrition model, gestational supplementation with SCFAs prevents placental growth restriction and vascular insufficiency. These findings highlight the important role of host-microbial symbioses during pregnancy and reveal that the maternal gut microbiome promotes placental growth and vascularisation in mice.
What do we already know about this subject?
Recent studies have highlighted the significant influences of the maternal microbiome on the development of the offspring during the prenatal period 2, but we still do not understand exactly how the maternal microbiome influences maternal/ foetal health during pregnancy. The highly vascularised placenta, which facilitates the maternal/foetal exchange of nutrients and gas that sustain foetal development, lies at the intersection of the mother and foetus 3. The authors examined the effects of the maternal gut microbiome on placental development in mice, since it is a critical organ that shapes long-term health pathways.
What are the main insights from this study?
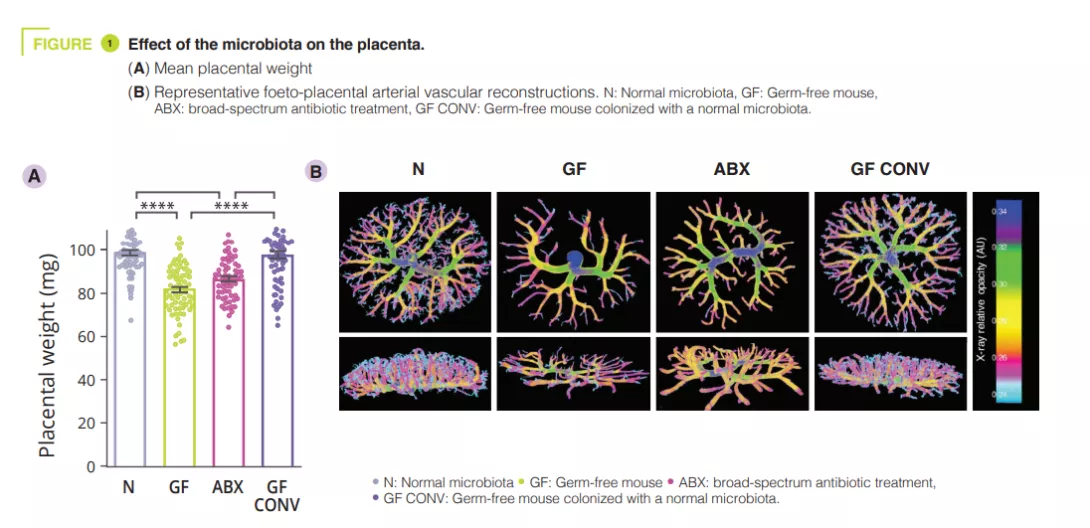
To determine effects of the maternal gut microbiome on placental development, the authors first reared pregnant mice as germ-free (GF) or having depleted the maternal gut microbiome by treating it with broad-spectrum antibiotics (ABX). Absence or depletion of the maternal gut microbiome resulted in reduced placental weight compared to GF controls colonised with a conventional microbiota (CONV) (figure 1). Consistent with the reductions in placental weight, maternal microbiome deficiency led to reductions in total placental volume, along with reduced volume and tissue density in the placental labyrinth - the primary site for maternal-foetal exchange. In addition to maternal ABX-induced placental pathophysiology, the authors observed corresponding decreases in foetal weight and volume. Reduced vascular volume and surface area was observed in the foeto-placental vascularisation of microbiota-deficient mothers, with a visible decrease in vascular branches, as compared to controls (figure 1).This suggests that the maternal microbiome instructs vascular development at critical gestational time points. Given that the maternal microbiome regulates many circulating metabolites, the authors postulated that foeto-placental vascularisation insufficiencies could be linked to the microbiota and could result from key metabolite alterations in foetal circulation. The authors looked specifically at the role of short-chain fatty acids (SCFAs). SCFAs are produced by bacterial carbohydrate fermentation and were observed to be significantly decreased in the maternal and foetal serum from microbiota-deficient mothers.
On the basis of previous research demonstrating that maternal supplementation with SCFAs leads to the direct transfer of SCFAs from maternal circulation to foetal circulation, the authors treated ABX mothers with SCFA-supplemented water or control water. The supplementation strategy significantly increased butyrate and propionate concentrations in foetal whole blood. Treating mothers with SCFAs increased placental weight and corrected alterations in placental growth in ABX mothers to levels comparable to those of controls, with corresponding increases in total placental and labyrinth volumes. Human umbilical vein endothelial cells (HUVECs) were then treated with SCFAs at physiological concentrations. The SCFAs acetate and propionate significantly increased HUVEC branching length compared to vehicle controls, whereas the signal with butyrate was less clear. This effect was dependent on free fatty acid receptors 2 and 3 (FFAR2 and FFAR3). In the context of maternal malnutrition induced by protein restriction, maternal supplementation with SCFAs was sufficient to restore total placental weight and volume and increase foeto-placental vascularisation.
What are the consequences in practice?
This study demonstrates the role of maternal intestinal microbiota in physiology, particularly in placental vascularisation. Placental vascular deficiencies are associated with reduced foetal weight, pre-eclampsia and, in adulthood, an increased risk of a number of diseases. Actions targeting the microbiota to promote SCFA production, primarily through nutrition, could play a protective role.
- Overall, the data reveals the key role of the maternal gut microbiome in promoting placental growth and development
- The maternal microbiome is necessary for the optimal development of foeto-placental vascularisation
- SCFAs promote placental growth and vascular development, even in conditions of maternal malnutrition
CONCLUSION
This study reveals that the metabolic functions provided by the maternal gut microbiome during pregnancy play an integral part in supporting placental growth and vascularisation in mice. Gaining a better understanding of how the maternal gut microbiome affects the structure and function of the placenta could lead to the development of new approaches to promote maternal and foetal health and reduce the risk of chronic diseases.

