Ethnicity associations with food sensitisation are mediated by gut microbiota development in the first year of life
Commented article - Children's section
By Pr. Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Comments on the original article by Tun HM et al. Gastroenterology 2021 [1]
Increasing evidence supports the role of early-life gut microbiota in the development of atopic diseases, but ecological changes to gut microbiota during infancy related to food sensitisation remain unclear. The authors sought to characterise and associate these changes with the development of food sensitisation in children. In this observational study, the authors used 16S rRNA sequencing to characterise the composition of 2,844 faecal microbiota in 1,422 Canadian full-term infants. Atopic sensitisation outcomes were measured by skin prick tests at the ages of 1 year and 3 years. Four developmental trajectories of gut microbiota were identified shaped by birth mode and by ethnicity. This study established a link between persistence of low Bacteroides abundance in the gut throughout infancy and peanut sensitisation in childhood. It is the first study to show a mediation role for infant gut microbiota in ethnicity-associated development of food sensitisation.
What do we already know about this subject?
The number of children with food allergies is increasing rapidly, currently representing 28% of children aged 1-5 years in the United States. The development of the gut microbiota (GM) in the first months of life may be involved in this sensitisation to food allergens [2]. Many factors influence the establishment of GM, such as the mode of delivery (caesarean versus vaginal), type of breastfeeding (breast or formula) and use of antibiotics [3, 4]. A recent study showed that GM structure also varied widely between different ethnic groups [5].
Transferring GM from healthy children to mice was shown to protect them from cow’s milk allergy. Low GM richness in young infants and a high ratio of Enterobacteriaceae/Bacteroidaceae (E/B) in early and late infancy are predictors of food allergen sensitisation [6].
What are the main insights from this study?
The study included 1,422 children from the CHILD (Canadian Healthy Infant Longitudinal Development) cohort. Prick tests were performed (inhalant and food allergens) at the ages of 1 and 3 years. Stool samples were collected in early (3.5 ± 0.9 months) and late (12.2 ± 0.3 months) infancy.
Atopy prevalence was 12% at 1 year and 12.8% at 3 years, with 9.5% and 5.8% of food sensitisation and 3.3% and 10.1% of sensitisation to inhalant allergens at ages 1 and 3 years, respectively.
Late infancy GM had lower beta-diversity and intra-individual variability compared to early infancy GM (p < 0.001). Late infancy gut microbiota were enriched with Bacteroides, Faecalibacterium, Lachnospira, Prevotella, unclassified Lachnospiraceae and unclassified Clostridiales, but depleted with Clostridium, Veillonella, Bifidobacterium and unclassified Enterobacteriaceae. The principal component analysis identified two clusters (C1 and C2, Figure 1). C1 was composed of 75.5% of early infancy samples and C2 of 63.7% of late infancy samples. These early and late infancy samples representing vaginal births without intrapartum antibiotic prophylaxis were of type C2, dominated by the genus Bacteroides (Figure 2).
The authors identified four trajectories according to the type of early and late infancy cluster: C1-C1, C1-C2, C2-C1 and C2-C2. The C1-C1 trajectory was more common among Asian than Caucasian infants (p < 0.05), as well as in atopic-risk children compared to the C2-C2 (OR 1.9; 95% CI 1.15-3.14) or C1-C2 (OR 2.38; 95% CI 1.43-3.96) trajectory. Infants in the C1-C1 trajectory were twice as likely to have food sensitisation at the age of 3 years compared to those in the C2-C2 trajectory (OR 2.34; 95% CI 1.20- 4.56) and C1-C2 trajectory (OR 2.60; 95% CI 1.33-5.09), especially to peanuts (vs C2-C2 = OR 2.82; 95% CI 1.13-6.01 and vs C1-C2 = OR 2.01; 95% CI 0.85-4.78) (Figure 3). Children who had not acquired peanut sensitisation at the age of 3 years had persistently higher levels of Bacteroides
(p = 0.044), lower levels of unclassified Enterobacteriaceae (p = 0.001) and a lower E/B ratio (p = 0.013) throughout childhood.
The C1-C1 trajectory mediated the risk of food and peanut sensitisation in Asian children. The association was even high for peanuts (OR 7.87; 95% CI 2.75-22.55). Infants in the C1-C1 trajectory were more often colonised with C. difficile. These same children, with both the C1-C1 characteristic and colonised with C. difficile, had an extra risk of food (OR 5.69; 95% CI 1.62-19.99) and peanut (OR 5.89; 95% CI 1.16-29.87) sensitisation.
Finally, microbiota of the C1-C1 trajectory had a deficiency in sphingolipid metabolism and glycosphingolipid biosynthesis-related functions.
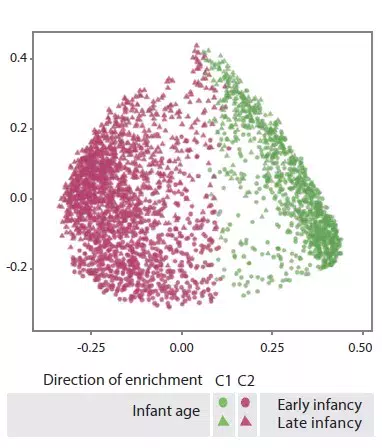
FIGURE 1
C1 and C2 gut microbiota clusters (principal component analysis).
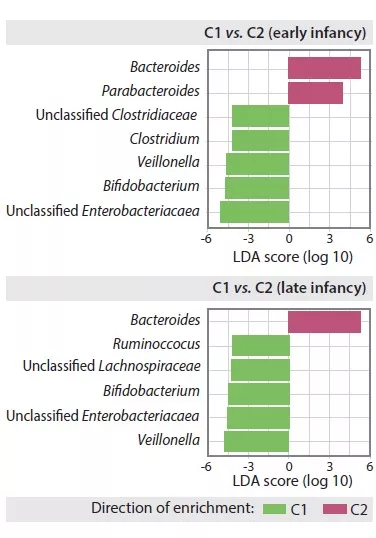
FIGURE 2
Composition of gut microbiota in early and late C1 and C2 clusters in infants.
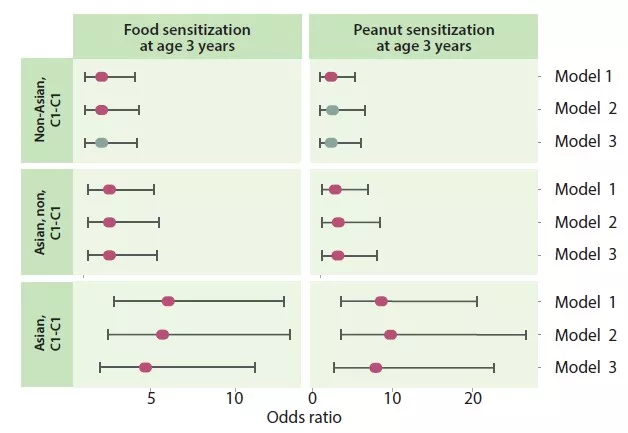
FIGURE 3
Food and peanut sensitisation at the age of 3 years according to the C1-C1 trajectory and Asian origin of the mother
What are the consequences in practice?
This study allows us to envisage GM-targeted therapies for food allergies in infants, either as a preventative or therapeutic option.
Conclusion
This study showed different developmental trajectories of the gut microbiota in the first year of life. It confirms the impact of the birth mode on gut microbiota. Persistently low levels of Bacteroides were associated with a risk of food sensitisation, particularly in neonates of Asian mothers or those colonised with C. difficile.
Key points
- During the development of the gut microbiota in the first year of life, persistently low levels of Bacteroides increase the risk of food sensitisation, especially to peanuts
- This risk is increased in neonates of Asian mothers
1 Tun HM, Peng Y, Chen B, et al. Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Gastroenterology 2021 ; 161 : 94-106.
2 Zhao W, Ho HE, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol 2019 ; 122 : 276–82.
3 Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010 ; 107 : 11971–5.
4. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016 ; 8 : 343ra82.
5 Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence specific variations in human microbiome composition and diversity. Front Microbiol 2017 ; 8 : 1162
6 Feehley T, Plunkett CH, Bao RY, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nature Medicine 2019 ; 25 : 448.