I Cultivated Enterococcus faecium B6 from children with obesity promotes non-alcoholic fatty liver disease by the bioactive metabolite tyramine
COMMENTED ARTICLE Children’s section
By Pr. Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France
Sources
This article is based on scientific information
Sections

About this article
Comments on the article by Wei et al. (Gut Microbes 2024) [1]
This article explores the relationship between the gut microbiota and non-alcoholic fatty liver disease (NAFLD) in children with obesity, in the context of the global increase in childhood obesity and this disease. The methodology is based on multi-omics analyses and studies of cohorts of children, combined with in vitro and in vivo experiments. The researchers discovered that Enterococcus faecium B6 isolated from these children promoted NAFLD via tyramine production, leading to lipid accumulation, inflammation and liver fibrosis. These findings validate the causal role of this bacterium in NAFLD progression and pave the way for therapeutic approaches based on microorganisms and/or their metabolites.
What do we already know about this subject?
Due to the obesity pandemic, metabolic overload diseases (e.g. NAFLD) have become the main type of liver injury ranging from steatosis, to NASH (non-alcoholic steatohepatitis) and even cirrhosis. NAFLD is now affecting an increasing number of children.
The pathophysiology of NAFLD is complex, but the gut microbiota is thought to play an important role. The effects of the gut microbiota could be mediated by various metabolites, including tyramine.
What are the main insights from this study?
In the first part of the study 156 children with obesity aged between 6 and 18 were enrolled, including 78 with NAFLD and 78 with isolated obesity. The two groups differed in terms of liver and metabolic parameters. Microbial alpha diversity was lower in the NAFLD group. The abundance of Enterococcus, Escherichia, Klebsiella, Dialister and Enterobacter was higher in the NAFLD group, while Faecalibacterium, Eubacterium_eligens_group, Roseburia, Fusicatenibacter, Clostridium, Coprococcus and Parasutterella abundance was lower. Enterococcus was correlated with serum levels of ALT, ASAT, triglycerides and total cholesterol.
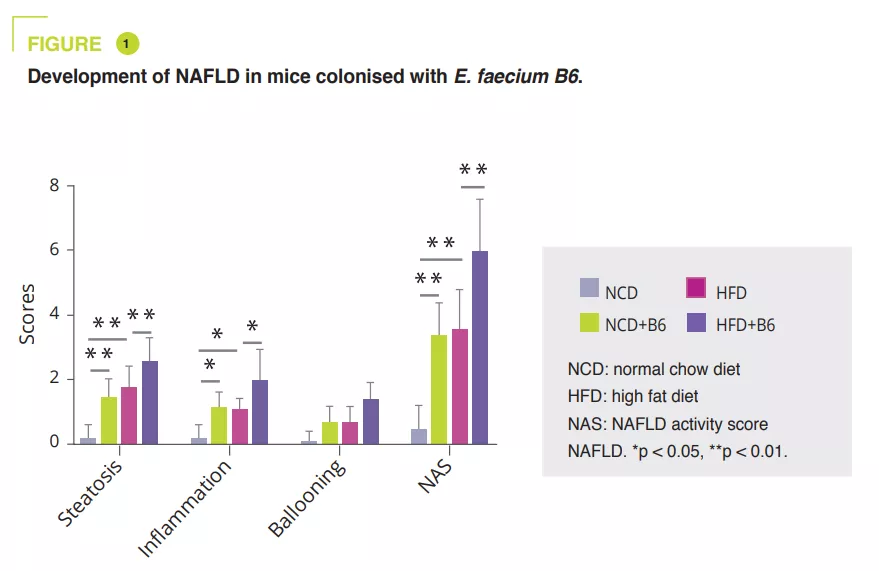
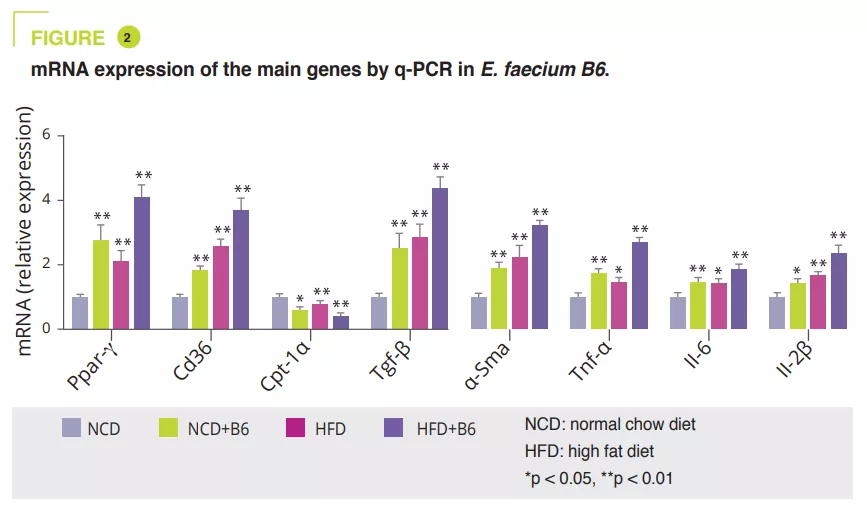
E. faecium B6 in the cell culture obtained after isolating bacterial strains from NAFLD children with obesity showed a lipid-accumulating ability. A murine study compared a normal (NCD) or a fat-enriched (HFD) diet, with or without B6, for 12 weeks. Although E. faecium B6 had no effect on body weight, it aggravated overload-related liver damage, both biologically and histologically (figure 1). Transcriptomic analysis revealed that E. faecium B6 modified gene expression in lipid metabolism and inflammation and fibrosis, such as PPAP, chemokine, NF-kB and TGF-β signalling pathways, in linoleic acid metabolism. Regarding lipid metabolism, mRNA and protein expression of PPAR and CD36 were increased, while that of CPT-1a decreased. The authors reported increased expression of inflammatory cytokines (TNF-α, IL-6, IL-1β) and proteins involved in fibrosis (TGF-β and α-SMA) (figure 2).
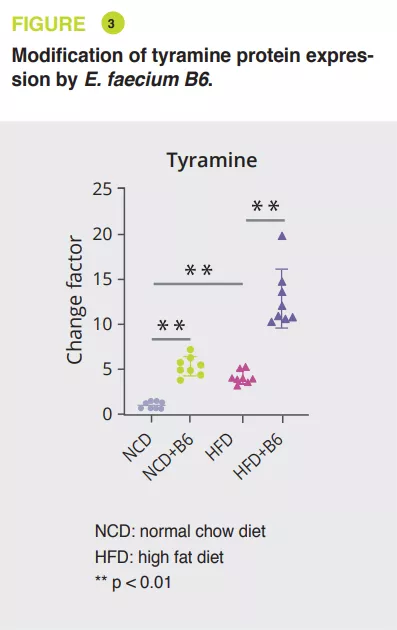
Using mice serum, a broad, non-targeted analysis via tandem mass spectrometry showed that E. faecium B6 increased or decreased metabolites, when on NCD (30 and 85) or HFD (18 and 45) diets. The most notable change was the increase in tyramine (figure 3). Further analyses have suggested that E. faecium B6 is able to produce tyramine. In addition, treating mice on the NCD or HFD diet with tyramine reproduced the development of NAFLD, without effect on weight, in a similar fashion as that observed with E. faecium B6, and similarly altered the expression of genes encoding PPAR , CD36, CPT-1a, TNF-α, IL-6, IL-1β, TGF-β and α-SMA.
Finally, the authors corroborated these findings in 123 NAFLD children with obesity and in 123 controls. Levels of E. faecium B6 and the gene encoding tyramine (mfnA) were higher in the NAFLD group. These levels were correlated with biological markers of NAFLD (AST, ALAT, triglycerides, total cholesterol and LDL) and inflammatory cytokines (TNF-α, IL-6, IL-1β).
What are the consequences in practice?
This study confirms the importance of the gut microbiota in the development of NAFLD and paves the way for new therapeutic strategies. In addition to Enterococcus faecium B6 and tyramine, PPAR y could play a central role in linking lipid accumulation, inflammation and fibrosis.
- E. faecium B6, a bacterium isolated from NAFLD children with obesity, exacerbates the disease by producing a bioactive metabolite, tyramine
- E. faecium B6 and tyramine reproduce NAFLD in a similar way in mice on normal and high-fat diets
CONCLUSION
This study identified Enterococcus faecium B6 as a strain that promotes the development of NAFLD in children with obesity. This bacterium produces a bioactive metabolite, tyramine, which mediates these effects by activating the PPARy signalling pathway




