When the bacteria in the intestinal microbiota store medicines
The bioaccumulation of medicines by the intestinal bacteria changes their availability and the bacterial secretion of metabolites. With, into the bargain, possible dysbiosis and implications in terms of pharmacokinetics, adverse events and responses to medicines.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
About this article
We know: medicines have an influence on the intestinal microbiota. But did you that there are also interactions in the other direction? With, into the bargain, a positive or negative effect on the efficacy of medicines. Take for example lovastatin and sulphasalazine, they are chemically transformed by intestinal bacteria into their active forms, whereas digoxin is inactivated by bacterial metabolism. More than 100 molecules have recently been notified as being affected by the intestinal microbiota in this way. And according to the results from a research team, the mechanisms in play are far from limiting themselves to a single biotransformation...
Biotransformation and above all bioaccumulation
The study in question has sifted through the interactions between 25 representative strains of human intestinal bacteria and (sidenote: 12 molecules administered orally and 3 controls: digoxin (highly specific interaction with Eggerthella lenta), metronidazole and sulphasalazine, medicines which are known to be metabolised by several intestinal bacteria ) . The results? The in vitro cultures of 15*25= 375 bacteria-medicines pairs show 70 bacteria-medicines interactions, 29 of which (18 species, 7 medicines) were unknown until now. Above all, 12 of these 29 new interactions are explained by biotransformation phenomena. All the other cases, i.e. 17 interactions (14 species, 4 medicines), are based on bioaccumulation: the bacteria store the medicine in their cells without modifying it, and in most cases without any effect on the growth of the bacteria. Amongst the medicines that are exclusively bioaccumulated, (sidenote: Duloxetine Antidepressant, a selective serotonin and norepinephrine reuptake inhibitor ) and the antidiabetic, rosiglitazone, are noted. However, bioaccumulation is not routine: some molecules (montelukast, roflumilast) can be bioaccumulated by some bacterial species and biodegraded by others.
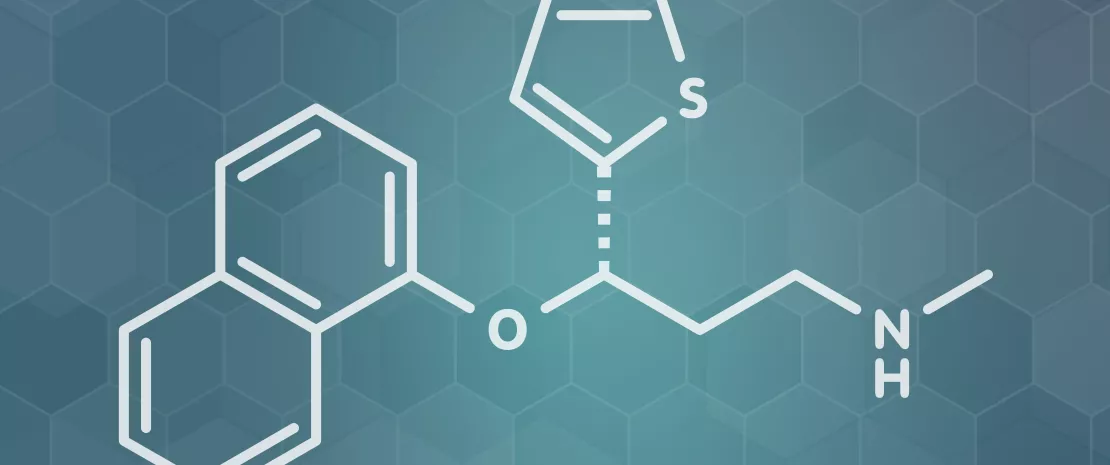
The case of duloxetine
As an example, the team studied the bioaccumulation of duloxetine more closely. Duloxetine binds to numerous bacterial enzymes and modifies the secretion of metabolites by the bacteria concerned. When it is tested in a microbial community of 5 bacterial species containing both accumulating and non-accumulating bacteria, duloxetine greatly changes the composition of the community. In fact, apart from the sequestration of this medicine which is harmful for some bacteria, the bioaccumulation of this medicine causes the secretion of metabolites by some species (Streptococcus salivarius) which will serve as a substrate to nourish others (Eubacterium rectale), thus greatly increasing their abundance. In this way medicines intended for humans seem capable of modulating the intestinal microbial communities, not only by direct inhibition but also by creating synergies of cross-feeding. The results were confirmed on the model, Caenorhabditis elegans: bioaccumulating bacteria reduce the effect of duloxetine on the movement of this worm.
The results of this study indicate that the bioaccumulation of medicines within the intestinal bacteria would modify their availability and bacterial metabolism. As this could cause individual repercussions within the composition of the intestinal microbiota, and also for the pharmacokinetics and the drug response. The authors suggest routinely establishing search for reciprocal interactions between bacteria and medicines so as to estimate the side effects in the best possible way.