Using recombinant endolysins to treat bacterial vaginosis
A study has shown that by using recombinant endolysins encoded on a prophage it is possible to eliminate the bacterial biofilm responsible for bacterial vaginosis without damaging the beneficial bacteria of the vaginal microbiota. These are promising results.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
About this article
35% Only 1 in 3 women know that bacterial vaginosis is associated with an imbalance in the vaginal microbiota
Bacterial vaginosis is a quite common disorder in women of reproductive age, with a prevalence estimated at 10%–30% worldwide. It is associated with an increased risk of infertility and complications during pregnancy. It is also a risk factor for contracting sexually transmitted diseases. The condition is characterized by an imbalance of the vaginal microbiota and a biofilm formed on the vaginal epithelium, which is initiated and dominated by Gardnerella bacteria. This biofilm is frequently refractory to antibiotic treatment. Antibiotics are effective in quickly reducing symptoms but are associated with a recurrence rate of up to 60% within six months of treatment. A new study has investigated (sidenote: Endolysins Bacteriophage enzymes that lyse the bacterial wall, allowing the release of phages. ) of the type 1,4-beta-N-acetylmuramidase encoded on Gardnerella (sidenote: Prophages Bacteriophage genomes integrated into the host genome. (Saussereau and Debarbieux 2012) ) as an alternative treatment.
Bactericidal effect 10 times higher than wild type
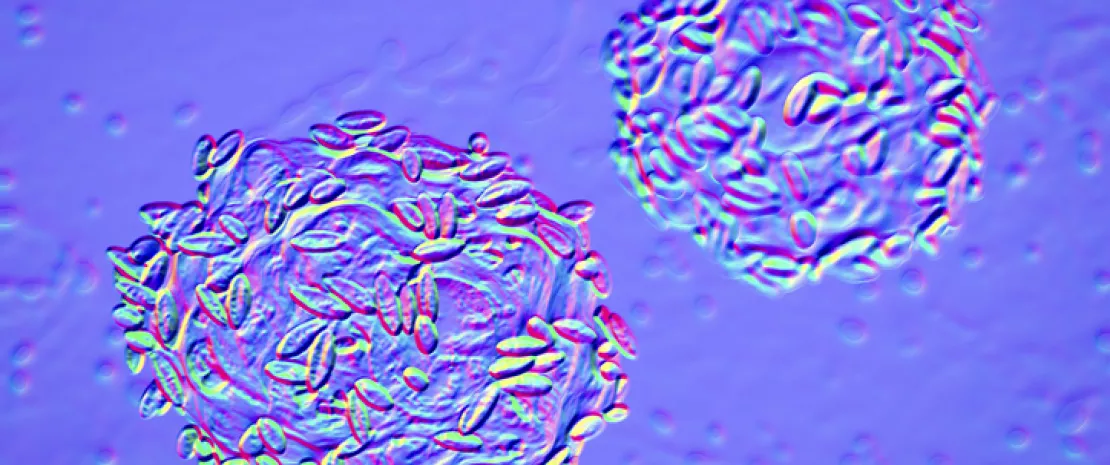
To this end, the authors generated several engineered endolysins via domain shuffling. They compared their bactericidal activity on Gardnerella strains to that of wild-type endolysins. The bactericidal activity of the recombinant endolysins was 10 times that of any wild-type enzyme. When tested against a panel of 20 Gardnerella strains (from (sidenote: G. vaginalis, G. leopoldii, G. piotii and G. swidsinskii ) ), the most active endolysin, called PM-477, showed superior efficacy compared to the antibiotics tested (metronidazole, tinidazole, clindamycin). Furthermore, PM-477 had no effect on beneficial lactobacilli or other species of vaginal bacteria. According to the authors, PM-477 is highly selective for Gardnerella and kills strains of each of the four main species without affecting beneficial lactobacilli or other species typical of the vaginal microbiota. The effect of PM-477 was confirmed by microscopy in mixed cultures of Gardnerella and lactobacilli. PM-477 (at 460 µg/mL for 5 h) lysed G. vaginalis and G. swidsinskii cells in monoculture, but also selectively lysed them in mixed cultures alongside lactobacilli without affecting the latter.
Efficacy in patient samples
To go further and analyze the efficacy of PM-477 in a physiological environment closely resembling the in vivo situation, the researchers treated vaginal swabs from 15 bacterial vaginosis patients and analyzed them by fluorescence in situ hybridization (FISH). They showed that in 13 of the 15 cases, PM-477 eradicated the Gardnerella bacterium and physically dissolved the biofilms without affecting the vaginal microbiota. For the authors, endolysins are a promising therapeutic alternative to antibiotics for the treatment of bacterial vaginosis. This is a significant finding since antibiotics are frequently a cause of recurrence and resistance in the treatment of the disease.