Zoom on the risks of IBD
There is an increased risk of Inflammatory Bowel Disease (IBD) in young children who have been exposed intrapartum to antibiotics. The physiological disruptions caused by dysbiosis, in particular within the intestinal mucosa and the immune system, facilitate the development of this type of rare inflammatory diseases.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
EXPOSURE TO ANTIBIOTICS AND EARLY IBD
The excess weight issue underlines the complexity in elucidating the impact of dysbiosis in some medical fields. Causal relations have been more detailed in the gastroenterology field, where a link has been established between the composition of the intestinal microbiota and IBD. This connection leads researchers to increasingly take dysbioses into account when attempting to understand disorders with poorly identified etiology, especially ulcerative colitis (UC) and Crohn’s Disease in young children (under six years of age), whose global incidence is increasing regularly. As the cause of this progression cannot be found in known genetic and environmental factors, intestinal weakness associated with disruptions in the microbiota has been suggested.
TWOFOLD HIGHER RISK OF IBD AFTER INTRAPARTUM ANTIBIOTIC TREATMENT
Swedish researchers explored this hypothesis by studying a cohort of 827,239 children born between 2006 and 2013.3 This was an extensive analysis based on crosschecking the Swedish National Registers of births, patients and drug prescriptions. In total, 17% of subjects were exposed intrapartum to antibiotics (including 5% repeatedly) and 65% after birth, most of them more than once (7 out of 10). Fifty-one children were affected by Crohn’s disease or UC. Compared to the control population, children exposed to antibiotics during pregnancy had an elevated risk (aHR4 = 1.93) of developing early IBD.
FETAL IMPACT
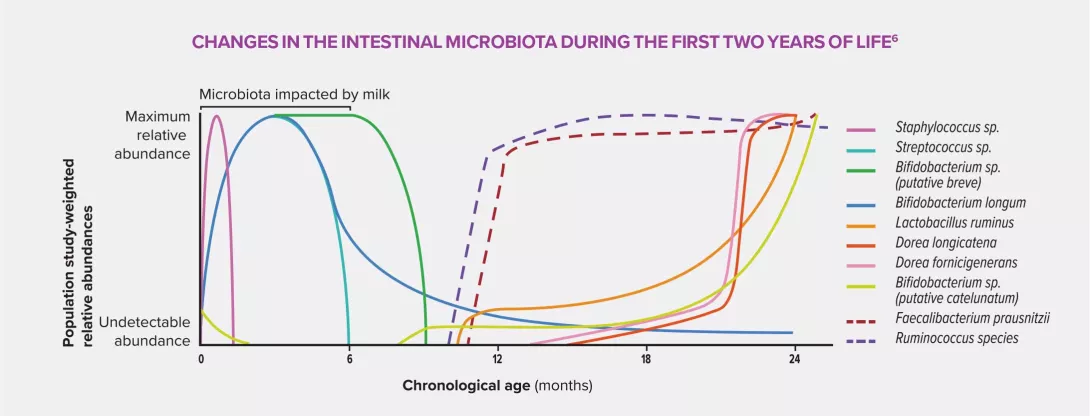
Intrauterine exposure to antibiotics is believed to trigger a disruption of early bacterial colonization in children, characterized by a low concentration of commensal bacteria, in particular Faecalibacterium prausnitzii and Ruminococcaceae, and an increase in pathogenic bacteria. This dysbiosis could cause significant physiological changes due to the interaction between microbiota and host through production of SCFA (particularly butyrate), induction of the immune system of the intestinal mucosa, stimulation of the local nervous system and maintenance of the intestinal barrier function.5 These are all malfunctions which are liable to trigger inflammatory disorders.
IMPROVING ANTIBIOTIC PRESCRIPTION TO PRESERVE THE MICROBIOTA
The data mentioned above and those derived from a growing number of studies and publications show that a diversified microbiota displaying a high commensal/pathogenic bacteria ratio contributes to an adequate development in children by limiting the risk of occurrence of some diseases, especially metabolic or inflammatory. This observation should not lead to a dismissal of antibiotic therapy, whose efficacy and benefits prove invaluable in many cases, as emphasized by all healthcare providers. However, optimizing prescriptions, spectrum of the drugs employed, duration of treatment and modes of administration are good ways of limiting the impact of antibiotics and the transfer of resistance to the intestinal microbiota in order to preserve child health in the short and long term.
4 Ajusted Hazard Ratio
5 Short-chain fatty acids. They are products of carbohydrate fermentation (organic anions and saturated fatty acids) carried out by anaerobic bacteria in the colon.
6 Rasmussen SH, Shrestha S, Bjerregaard LG, et al. Antibiotic exposure in early life and childhood overweight and obesity: A systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(6):1508-1514.








