The effect of antibiotics on infant mycobiota
A single course of antibiotics would be sufficient to permanently alter the mycobiota of infants. How? Presumably through their bacterial microbiota, with possible long-term effects on their health. Explanation follows.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
About this article
We already know that taking antibiotics can disrupt gut bacteria. It seems that they are not alone. While most studies focus on the impact of antibiotics on bacteria, few assess the effect on other microorganisms, such as fungi, whose role should not be underestimated. Fungal microbiota or mycobiota dysbiosis has been associated with various disorders and diseases (IBD, celiac disease and colorectal cancer). However, this mycobiota develops progressively in the first few years of life in the same way as bacterial colonization, depending on the mode of delivery, diet or even possible antibiotic treatments.
A study on 37 antibiotic-naive infants
To learn more, researchers followed 37 children for 9.5 months with an average age of 2 months who had never received antibiotics. These infants were hospitalized with respiratory syncytial virus (RSV) infections. Stool samples were taken before, during and after one to four antibiotic treatments (amoxicillin, macrolides) that were prescribed to 21 of them due to complications (otitis media, etc.). The remaining 16 untreated children made up the control group.
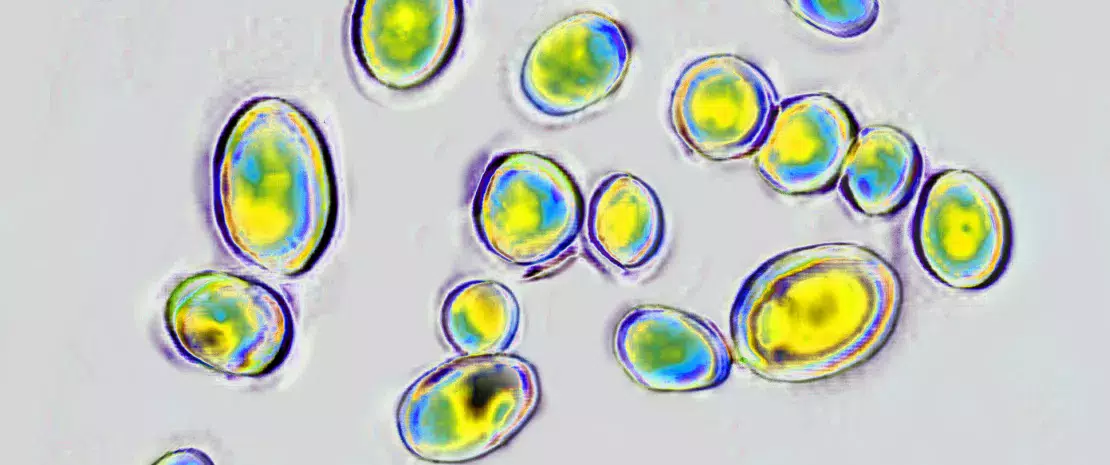
At the time of hospitalization (i.e. before treatment), the mycobiome of the 37 children was overwhelmingly dominated by Saccharomyces and to a much lesser extent Malassezia, Candida and Cladosporium.
More than one in four children In Finland, more than one in four children had already received antibiotic treatment before the age of 5 in 2019.
10 days Within 10 days of birth, an infant's gut microbiota is colonized by fungi.
2 years By the age of 2, a child’s gut mycobiota already shows similarities to that of an adult.
More Candida, diversity and abundance
One to two days after the start of the antibiotic treatment, the abundance of Candida greatly increased in infants on amoxicillin, at the expense of Saccharomyces. An overabundance of Candida continued to be observed more than 6 weeks after the start of treatment.
In addition, antibiotics, which are known to induce a collapse in the diversity and abundance of bacterial microbiota, were associated with an increase in the abundance and diversity of the mycobiota. This appeared within 3 to 5 days of the start of treatment and persisted well beyond that time (> 6 weeks) with the difference most marked in the macrolide group.
Antibiotics are an extraordinary scientific discovery that saves millions of lives but their excessive and inappropriate use has now raised serious concerns for health, notably with antibiotic resistance and microbiota dysbiosis. Let’s take a look at this dedicated page:
The ambivalent role of antibiotics
Are there bacteria that play a regulatory role?
These results strongly suggest that gut bacteria regulate mycobiota on an ongoing basis. This regulation takes the form of competition for nutrient sources through the production of antifungal compounds by bacteria and, conversely, antibacterial compounds by fungi. As soon as bacteria are affected by an antibiotic, their regulatory role is altered and certain fungi, particularly Candida, are given free rein to develop. As such, gut mycobiota dysbiosis after a single antibiotic treatment could, together with an alteration of gut bacteria, induce the long-term effects of antibiotics on human health.
What is the World AMR Awareness Week?
Each year, since 2015, the WHO organizes the World AMR Awareness Week (WAAW), which aims to increase awareness of global antimicrobial resistance.
Held on 18-24 November, this campaign encourages the general public, healthcare professionals and decision-makers to use antimicrobials carefully, to prevent the further emergence of antimicrobial resistance.
Recommended by our community

"Now that's an interesting take" - Jesús Jacinto (From Biocodex Microbiota Institute on X)






